M86804
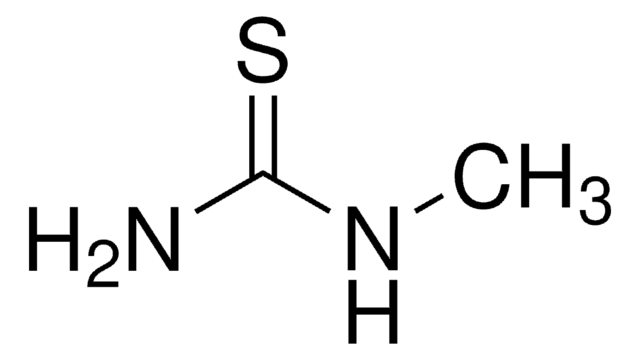
N-Methylurea
97%
Synonym(s):
1-Methylurea, N-Methylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3NHCONH2
CAS Number:
Molecular Weight:
74.08
Beilstein:
878189
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
crystals
SMILES string
CNC(N)=O
InChI
1S/C2H6N2O/c1-4-2(3)5/h1H3,(H3,3,4,5)
InChI key
XGEGHDBEHXKFPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L W Yousef et al.
Biochimica et biophysica acta, 984(3), 281-288 (1989-09-18)
Permeability coefficients (P) measured at various penetrant concentrations (C) by the perturbation method can be plotted to distinguish simple diffusion, simple pore kinetics and simple carrier kinetics as follows: for simple diffusion, 1/P = constant; for a simple pore, 1/P
P Vaughan et al.
Carcinogenesis, 12(2), 263-268 (1991-02-01)
Many microorganisms exhibit an adaptive response to mutagenic alkylation damage. In Escherichia coli the response is regulated by the inducible Ada protein. A sensitive immunoassay employing two anti-Ada monoclonal antibodies has been developed here to monitor low levels of induction
Nuzhat Gull et al.
Journal of biochemistry, 141(2), 261-268 (2006-12-19)
We report that the presence of very low concentrations (<0.1 M) of urea, a widely used chemical denaturant, induces structure formation in the water-soluble globular protein human serum albumin (HSA) at pH 7. We have presented results suggesting an almost
L S Povarov et al.
Bioorganicheskaia khimiia, 16(4), 559-568 (1990-04-01)
Derivatives of antitumour anthracycline antibiotics containing N-methylurea moiety in the carbohydrate ring were obtained by the interaction of methyl isocyanate with daunorubicin, doxorubicin, carminomycin and daunorubicin derivatives, substituted at C-13 or C-14 positions. N-Nitrosation of these compounds yielded modified anthracycline
J Guzmán Rincón et al.
Mutation research, 412(1), 69-81 (1998-03-21)
The in vivo nitrosation capacity of third-instar larvae of Drosophila melanogaster was assessed using the wing somatic mutation and recombination test (SMART). Larvate derived from two different crosses, the standard cross (ST) and the high bioactivation cross (HB) both involving
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service