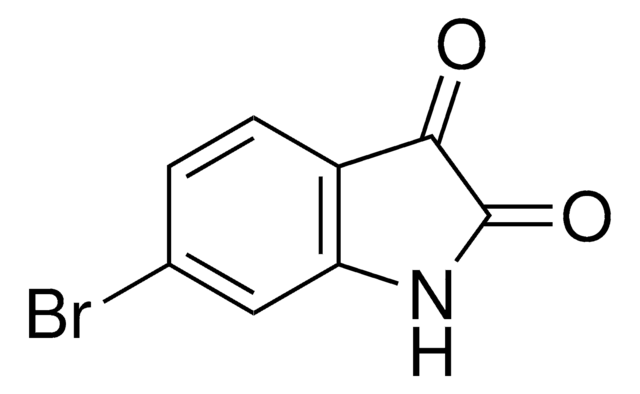
377406
3,4-Dimethoxy-3-cyclobutene-1,2-dione
99%
Synonym(s):
Dimethyl squarate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)2C4(=O)2
CAS Number:
Molecular Weight:
142.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
55-57 °C (lit.)
storage temp.
2-8°C
SMILES string
COC1=C(OC)C(=O)C1=O
InChI
1S/C6H6O4/c1-9-5-3(7)4(8)6(5)10-2/h1-2H3
InChI key
SZBNZTGCAMLMJY-UHFFFAOYSA-N
Related Categories
General description
3,4-Dimethoxy-3-cyclobutene-1,2-dione (Dimethyl squarate) is a cyclobutene derivative. It reacts with hydroxylamine derivatives to afford 3-hydroxyamino-4-methoxy-3-cyclobutene-1,2-diones.
Application
3,4-Dimethoxy-3-cyclobutene-1,2-dione (Dimethyl squarate) may be used in the synthesis of the following compounds:
- chiral squaramides, highly enantioselective catalyst for the Friedel-Crafts reactions of indoles
- 3-(hydroxyamino)-4-methoxy-3-cyclobutene-1,2-dione
- squarate derivatives of the O-SP-core antigens
- methyl squarate derivative of the Ogawa O-SP-core antigen
- o-quinodimethanes
- benzocyclobutenes
- quinones
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hydroxylamin-Derivate der Quadratsaure.
Zinner G, et al.
Arch. Pharm. (Weinheim), 318(11), 977-983 (1985)
Kerriann K Badal et al.
Cell reports, 26(3), 507-517 (2019-01-17)
Mechanisms that regulate the bi-directional transport of mitochondria in neurons for maintaining functional synaptic connections are poorly understood. Here, we show that in the pre-synaptic sensory neurons of the Aplysia gill withdrawal reflex, the formation of functional synapses leads to
Peng Xu et al.
Bioconjugate chemistry, 22(10), 2179-2185 (2011-09-09)
Bacterial O-SP-core antigens can be conjugated to proteins in the same, simple way as synthetic, linker-equipped carbohydrates by applying squaric acid chemistry. Introduction of spacers (linkers) to either O-SP-core antigens or protein carriers, which is involved in commonly applied protocols
The Journal of Organic Chemistry, 57, 6896-6896 (1992)
Yong Qian et al.
Chemical communications (Cambridge, England), 46(17), 3004-3006 (2010-04-14)
Chiral squaramides are highly enantioselective catalysts for Friedel-Crafts reaction of indoles with N-tosyl imines, affording 3-indolyl methanamine products in 85-96% yields and 84-96% ees.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service