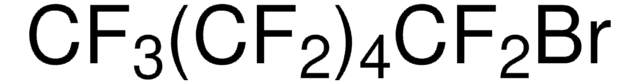274194
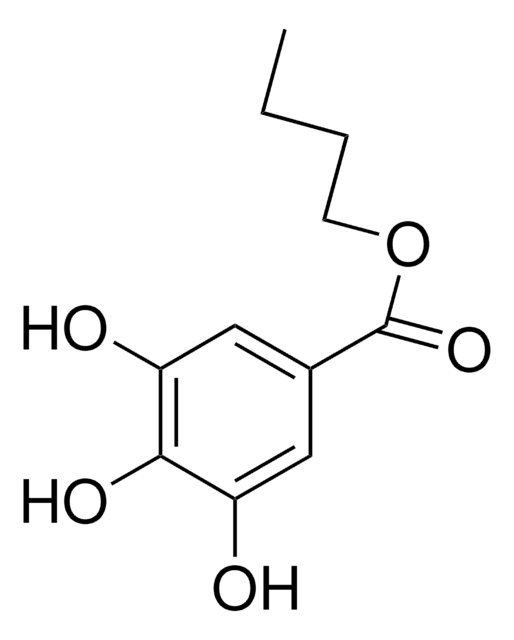
Methyl 3,4,5-trihydroxybenzoate
98%
Synonym(s):
Methyl gallate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)3C6H2CO2CH3
CAS Number:
Molecular Weight:
184.15
Beilstein:
2113180
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
mp
201-203 °C (lit.)
SMILES string
COC(=O)c1cc(O)c(O)c(O)c1
InChI
1S/C8H8O5/c1-13-8(12)4-2-5(9)7(11)6(10)3-4/h2-3,9-11H,1H3
InChI key
FBSFWRHWHYMIOG-UHFFFAOYSA-N
General description
Methyl 3,4,5-trihydroxybenzoate, also known as methyl gallate, is a derivative of gallic acid with three hydroxyl groups and an ester linkage, which contributes to its reactivity and solubility in organic solvents. It exhibits strong free radical scavenging activity, which is crucial in preventing oxidative stress in biological systems and enhancing the lifespan of materials. Its natural origin and low toxicity make it suitable for biomedical applications, particularly in drug delivery and tissue engineering.
Application
Methyl 3,4,5-trihydroxybenzoate can be used:
- As a precursor to synthesize nanocomposite drug delivery systems for lung cancer treatment. It has low toxicity and can induce apoptosis by generating free radicals.
- As a starting material to prepare norbornene-based main-chain polymers via ring-opening metathesis polymerization (ROMP), for biomedical uses to coatings and electronics.
- As an additive in the pretreatment of Sugarcane Bagasse for the removal of lignin from biomass and consequently the cellulose hydrolysis. This process helps the use of biomass as energy currency for various biotechnological processes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rayza Morganna Farias Cavalcanti et al.
3 Biotech, 8(6), 270-270 (2018-06-06)
One of the tannase isoforms produced by the fungus Aspergillus fumigatus CAS-21 under submerged fermentation (SbmF) was purified 4.9-fold with a 10.2% recovery. The glycoprotein (39.1% carbohydrate content) showed an estimated molecular mass of 60 kDa. Optimum temperature and pH for
Judith Delius et al.
PloS one, 12(9), e0184487-e0184487 (2017-09-09)
Nuclear magnetic resonance (NMR) spectroscopy is well-established in assessing the binding affinity between low molecular weight ligands and proteins. However, conventional NMR-based binding assays are often limited to small proteins of high purity and may require elaborate isotopic labeling of
J P Chu et al.
Journal of dentistry, 35(5), 383-387 (2007-01-02)
To evaluate the effect of compounds of Galla chinensis on the remineralisation of initial enamel carious lesions in vitro. Sixty bovine enamel blocks with early lesions were prepared and randomly divided into six treatment groups. The lesions were subjected to
Deepika Singh et al.
Molecules (Basel, Switzerland), 24(4) (2019-02-20)
Leea indica (Vitaceae) is a Southeast Asian medicinal plant. In this study, an ethyl acetate fraction of L. indica leaves was studied for its phytoconstituents using high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-microTOF-Q-MS/MS) analysis. A total of 31 compounds of different
James A G Crispo et al.
Biochemical and biophysical research communications, 393(4), 773-778 (2010-02-23)
Neurodegenerative disorders are a class of diseases that have been linked to apoptosis induced by elevated levels of reactive oxygen species (ROS). ROS activates the apoptotic cascade through mitochondrial dysfunction and damage to lipids, proteins and DNA. Recently, fruit and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service