254916
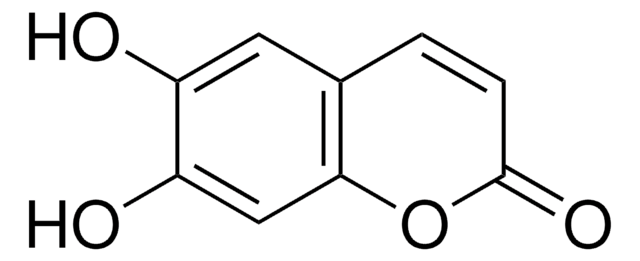
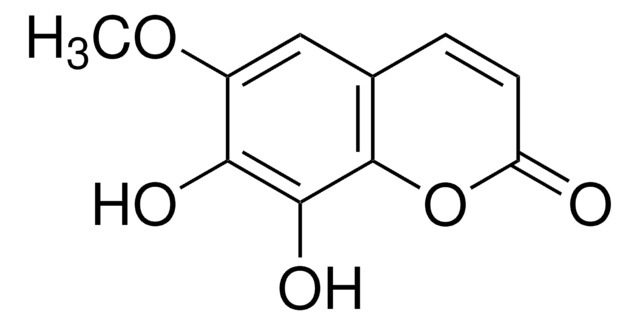
7,8-Dihydroxy-6-methoxycoumarin
98%
Synonym(s):
Fraxetin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8O5
CAS Number:
Molecular Weight:
208.17
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
powder
mp
230-231 °C (lit.)
SMILES string
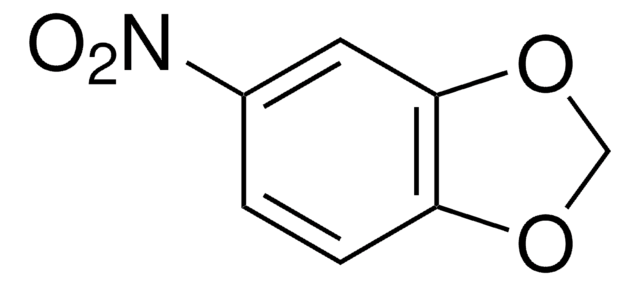
COc1cc2C=CC(=O)Oc2c(O)c1O
InChI
1S/C10H8O5/c1-14-6-4-5-2-3-7(11)15-10(5)9(13)8(6)12/h2-4,12-13H,1H3
InChI key
HAVWRBANWNTOJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
7,8-Dihydroxy-6-methoxycoumarin is a versatile compound known for its unique fluorescence properties and its role as a photoinitiator in polymerization processes. It exhibits high reactivity under UV light, making it an ideal candidate for applications in the polymerization industry, particularly in the formulation of coatings, adhesives, and inks. It is also used in biomedical applications, such as drug delivery due to its biocompatibility.
Application
7,8-Dihydroxy-6-methoxycoumarincan be used as a UV absorber in polymer formulations. Its ability to absorbultraviolet light helps in protecting polymers from degradation caused by UVradiation, thereby improving their longevity and stability.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
María Isabel Sánchez-Reus et al.
Neuroscience research, 53(1), 48-56 (2005-07-06)
Fraxetin belongs to an extensive group of natural phenolic anti-oxidants. In the present study, using a human neuroblastoma SH-SY5Y cells, we have investigated the protective effects of this compound on modifications in endogenous reduced glutathione (GSH), intracellular oxygen species (ROS)
Joana Terés et al.
Plant, cell & environment, 42(8), 2384-2398 (2019-04-25)
High soil carbonate limits crop performance especially in semiarid or arid climates. To understand how plants adapt to such soils, we explored natural variation in tolerance to soil carbonate in small local populations (demes) of Arabidopsis thaliana growing on soils
Po-Lin Kuo et al.
International immunopharmacology, 6(7), 1167-1175 (2006-05-23)
The survival of osteoblast cells is one of the determinants of the development of osteoporosis in patients with inflamed synovium, such as in rheumatoid arthritis (RA). By means of alkaline phosphatase (ALP) activity and osteocalcin ELISA assay, we have shown
María Francisca Molina-Jiménez et al.
Toxicology and applied pharmacology, 209(3), 214-225 (2005-05-21)
Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. Recently, it has been shown that fraxetin (coumarin) and myricetin (flavonoid) have significant neuroprotective effects against apoptosis induced by rotenone, increase the total glutathione levels in
Po-Lin Kuo et al.
Biological & pharmaceutical bulletin, 29(1), 119-124 (2006-01-06)
Fraxetin (7,8-dihydroxy-6-methoxy coumarin), a coumarin derivative, was investigated for its effects on differentiation of osteoblasts. By means of alkaline phosphatase (ALP) activity and osteocalcin ELISA assay, we have shown that fraxetin exhibits a significant induction of differentiation in two human
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service