1248218
USP
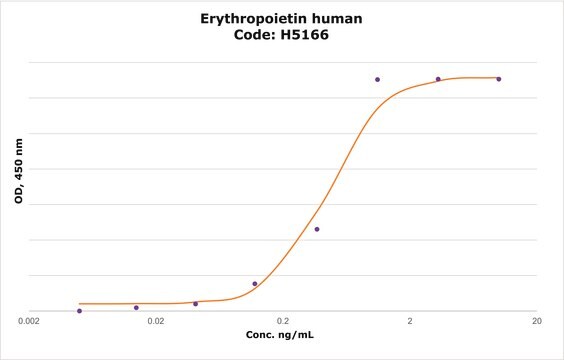
Erythropoietin
United States Pharmacopeia (USP) Reference Standard
About This Item
Productos recomendados
grado
pharmaceutical primary standard
Agency
USP
familia API
erythropoietin
Formulario
solid
fabricante / nombre comercial
USP
aplicaciones
pharmaceutical
pharmaceutical small molecule
Descripción general
Aplicación
Otras notas
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Contenido relacionado
Haga su pedido de una amplia gama de materiales de referencia primarios muy caracterizados para utilizar con las monografías de la USP-NF pensadas para el análisis de sustancias farmacológicas y formas de administración, excipientes farmacéuticos, ingredientes y suplementos alimentarios.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico