1205003
USP
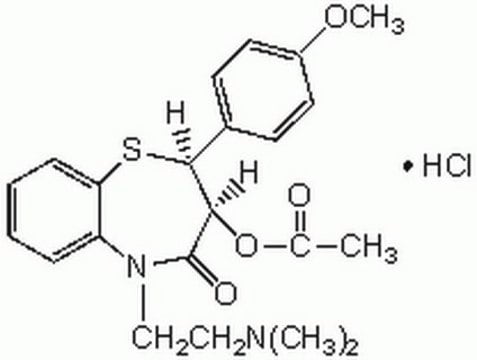
Diltiazem hydrochloride
United States Pharmacopeia (USP) Reference Standard
Sinónimos:
(+)-cis-Diltiazem hydrochloride, (2S,3S)-(+)-cis-3-Acetoxy-5-(2-dimethylaminoethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one hydrochloride, CRD-401
About This Item
Productos recomendados
grado
pharmaceutical primary standard
familia API
diltiazem
fabricante / nombre comercial
USP
aplicaciones
pharmaceutical (small molecule)
Formato
neat
cadena SMILES
Cl.COc1ccc(cc1)[C@@H]2Sc3ccccc3N(CCN(C)C)C(=O)[C@@H]2OC(C)=O
InChI
1S/C22H26N2O4S.ClH/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3;/h5-12,20-21H,13-14H2,1-4H3;1H/t20-,21+;/m1./s1
Clave InChI
HDRXZJPWHTXQRI-BHDTVMLSSA-N
Información sobre el gen
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Exploring the Effectiveness of Carboxymethylated and Crosslinked Albizia procera Gum in Diltiazem Hydrochloride Matrix Tablets: A Comparative Analysis. This study investigates the potential of carboxymethylated and crosslinked Albizia procera gum for use in sustained-release matrix tablets containing Diltiazem hydrochloride, highlighting its viability in pharmaceutical applications (Mukherjee S, Khanam J, 2024).
- Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. While primarily focused on gallic acid, this review mentions Diltiazem hydrochloride in the context of drug interactions and its implications in food safety and health (Xiang Z et al., 2024).
- Dual stimuli-responsive and sustained drug delivery NanoSensoGel formulation for prevention of cisplatin-induced ototoxicity. This research presents a novel NanoSensoGel that could be adapted for Diltiazem hydrochloride, enhancing drug delivery efficiency in clinical settings (Thakur NS et al., 2024).
- A novel potentiometric sensor based on ZnO decorated polyaniline/coal nanocomposite for diltiazem determination. This study develops a new sensor for the precise measurement of Diltiazem levels in pharmaceutical formulations, which is crucial for quality control and regulatory compliance (El Sayed GA et al., 2023).
- The Effect of Topical Nifedipine versus Diltiazem on the Acute Anal Fissure: A Randomized Clinical Trial. This clinical trial evaluates the effectiveness of Diltiazem hydrochloride in treating acute anal fissures, providing evidence of its beneficial applications in proctological disorders (Momayez Sanat Z et al., 2023).
Nota de análisis
Otras notas
Producto relacionado
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico