442475
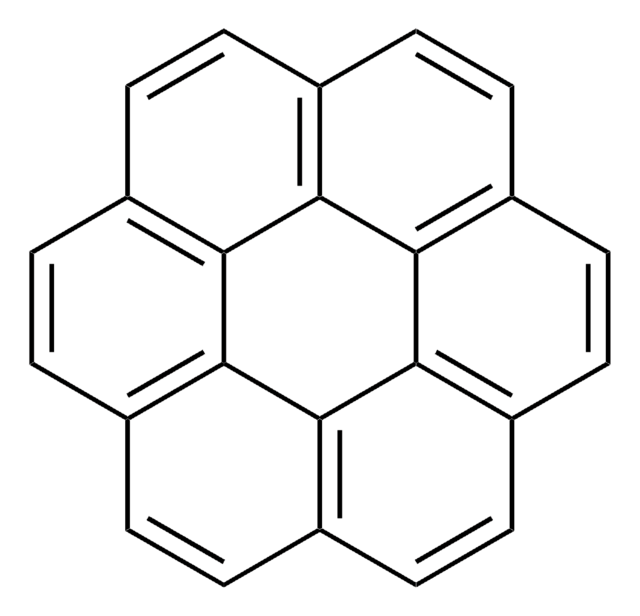
Benzo[e]pyrene
analytical standard
Sinónimos:
1,2-Benzpyrene, 4,5-Benzpyrene
About This Item
Productos recomendados
grade
analytical standard
CofA
current certificate can be downloaded
packaging
ampule of 25 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
177-180 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
food and beverages
personal care
format
neat
storage temp.
2-30°C
SMILES string
c1ccc2c(c1)c3cccc4ccc5cccc2c5c34
InChI
1S/C20H12/c1-2-8-16-15(7-1)17-9-3-5-13-11-12-14-6-4-10-18(16)20(14)19(13)17/h1-12H
InChI key
TXVHTIQJNYSSKO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![Benzo[a]pyrene ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)
![Benzo[e]pyrene 98%](/deepweb/assets/sigmaaldrich/product/structures/162/859/cd1f8e1f-2539-4f36-be04-8bad9d301215/640/cd1f8e1f-2539-4f36-be04-8bad9d301215.png)
![Benzo[a]pyrene-d12 98 atom % D](/deepweb/assets/sigmaaldrich/product/structures/962/892/b867e1bb-083c-4337-b499-36eae87f40ad/640/b867e1bb-083c-4337-b499-36eae87f40ad.png)

![Benzo[a]anthracene analytical standard](/deepweb/assets/sigmaaldrich/product/structures/351/486/b3ddf157-a732-4ef8-83f0-c70a53404cb2/640/b3ddf157-a732-4ef8-83f0-c70a53404cb2.png)
![Benzo[b]fluoranthene analytical standard](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)



![Benzo[ghi]perylene analytical standard](/deepweb/assets/sigmaaldrich/product/structures/154/740/c50ff1be-dfb4-4159-a98c-9cecf9206ad3/640/c50ff1be-dfb4-4159-a98c-9cecf9206ad3.png)