Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
R0395
Rapamycin
from Streptomyces hygroscopicus, ≥95% (HPLC), powder, mTOR inhibitor
Sinónimos:
23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine, AY 22989, Sirolimus
About This Item
Productos recomendados
Nombre del producto
Rapamycin from Streptomyces hygroscopicus, ≥95% (HPLC), powder
Nivel de calidad
Ensayo
≥95% (HPLC)
Formulario
powder
color
off-white
solubilidad
ethanol: 2 mM
DMSO: soluble
espectro de actividad antibiótica
fungi
yeast
Modo de acción
enzyme | inhibits
protein synthesis | interferes
temp. de almacenamiento
−20°C
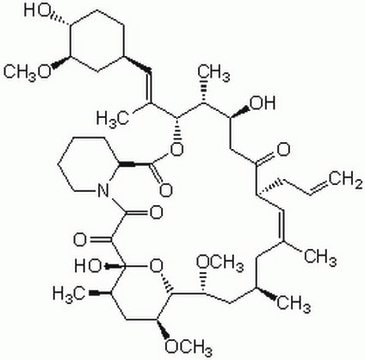
cadena SMILES
CO[C@@H]1C[C@@H](CC[C@H]1O)C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N4CCCC[C@H]4C(=O)O2)OC
InChI
1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1
Clave InChI
QFJCIRLUMZQUOT-HPLJOQBZSA-N
Información sobre el gen
human ... FKBP1A(2280)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- to treat BV2 cells or BV2-LC3 cells for autophagy assay[3]
- as an autophagy activator in the bone marrow mesenchymal stem cells (BM-MSCs) transfected with lentivirus carrier of small interfering RNA for FOXO3 (siFOXO3) to study the effects of Forkhead Box O3 (FOXO3) on the autophagy of BM-MSCs[4]
- to pre-incubate villi to inhibit the mammalian target of rapamycin complex 1 (mTORC1) signaling[5]
Acciones bioquímicas o fisiológicas
Características y beneficios
Otras notas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Carc. 2 - Repr. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
What are the extinction coefficient values for Product R0395, Rapamycin, solutions?
1 answer-
The E1% extinction coefficient values in 95% ethanol at 267 nm, 277 nm and 288 nm have been reported and are listed in the product information sheet (under Documents, above).
Helpful?
-
-
What is the solubility of Product R0395, Rapamycin?
1 answer-
Rapamycin is soluble in DMSO and in methanol (each at 25 mg/mL) and in ethanol (2 mM). It is insoluble in water. Additional solubility information is in the product information sheet (under Documents, above).
Helpful?
-
-
How should we store solutions of Product R0395, Rapamycin?
1 answer-
Rokaw, M.D. et al. J. Biol. Chem. 271, 32468 (1996) described storage of a 2 mM solution of rapamycin in ethanol at -70 °C which was diluted into serum-free media before use. Unless otherwise noted for other solvents, it may be best to prepare solutions fresh and protected from light. Please refer to our product information sheet at our website.
Helpful?
-
-
What is the shelf life of Product R0395, Rapamycin, as received?
1 answer-
The product has a recommended retest date of 2 years but the actual recommended retest date for each lot is on the Certificate of Analysis. We guarantee the quality of the product through this date for unopened bottles stored at -20 °C.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico