04646
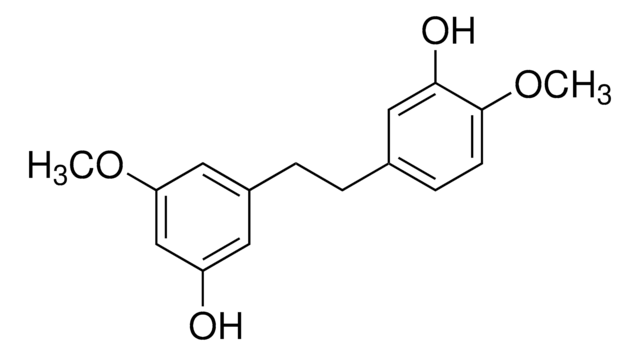
Hamamelitannin
≥98.0% (HPLC)
Sinónimos:
2-C-(Hydroxymethyl)-D-ribofuranose 2′,5-digallate, Hamamelofuranose 2′,5-digallate
About This Item
Productos recomendados
Quality Level
assay
≥98.0% (HPLC)
form
powder or crystals
application(s)
food and beverages
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
OC1O[C@H](COC(=O)c2cc(O)c(O)c(O)c2)[C@@H](O)[C@]1(O)COC(=O)c3cc(O)c(O)c(O)c3
InChI
1S/C20H20O14/c21-9-1-7(2-10(22)14(9)25)17(28)32-5-13-16(27)20(31,19(30)34-13)6-33-18(29)8-3-11(23)15(26)12(24)4-8/h1-4,13,16,19,21-27,30-31H,5-6H2/t13-,16-,19?,20-/m1/s1
InChI key
FEPAFOYQTIEEIS-IZUGRSKYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico