8.52110
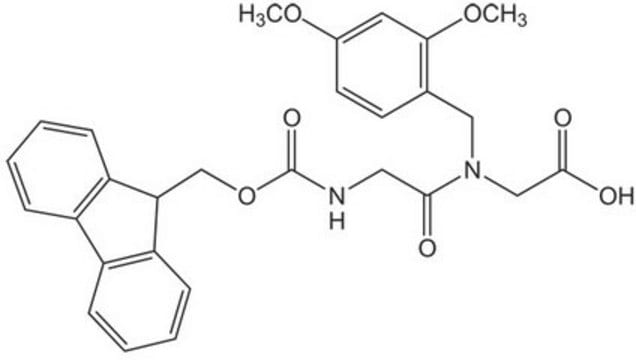
Fmoc-(Dmb)Gly-OH
Novabiochem®
Sinónimos:
Fmoc-(Dmb)Gly-OH, N-α-Fmoc-N-α-(2, 4-dimethoxybenzyl)-glycine
About This Item
Productos recomendados
Quality Level
product line
Novabiochem®
assay
≥94.0% (acidimetric)
≥98% (TLC)
≥99.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
-10 to -25°C
InChI
1S/C26H25NO6/c1-31-18-12-11-17(24(13-18)32-2)14-27(15-25(28)29)26(30)33-16-23-21-9-5-3-7-19(21)20-8-4-6-10-22(20)23/h3-13,23H,14-16H2,1-2H3,(H,28,29)
Inchi Key
UIDQSTVPYKMCEY-UHFFFAOYSA-N
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aggregation in Fmoc SPPS
Literature references
[1] M. El Haddadi, et al. (2000) J. Pept. Sci., 6, 560 (2000) J. Pept. Sci., 6, 560.
[2] K. Jauch, et al. in "Peptides 1996: Proc. 24th European Peptide Symposium", R. Ramage and R. Epton (Eds), Mayflower Scientific Ltd., 1996, pp 497.
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Purity (TLC(157B)): ≥ 98 %
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): passes test
Assay (acidimetric): ≥ 94.0 %
Water (K. F.): ≤ 1.00 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Protocolos
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico