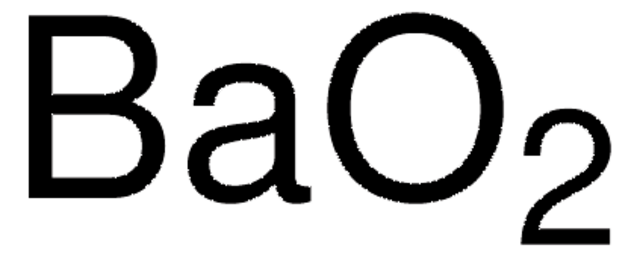1.01740
Barium peroxide
(anhydrous, heavy), Technipur®
Sinónimos:
Barium binoxide, Barium dioxide, Barium superoxide
About This Item
Productos recomendados
Quality Level
pH
12 (20 °C, 10 g/L in H2O, slurry)
mp
450 °C
density
4.96 g/cm3 at 20 °C
SMILES string
[Ba++].[O-][O-]
InChI
1S/Ba.O2/c;1-2/q+2;-2
InChI key
ZJRXSAYFZMGQFP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
BaO2 can also be used as a:
- Bleaching agent in industrial bleach applications.
- Catalyst to start an aluminothermic reaction.
- Oxidizer in various pyrotechnic mixtures.
- Green flare in tracer ammunition.
It can also find application in various other fields such as CO2 adsorption, disinfection, luminol-driven chemiluminescence, and wastewater treatment.
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
Storage Class
5.1A - Strongly oxidizing hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico