86872
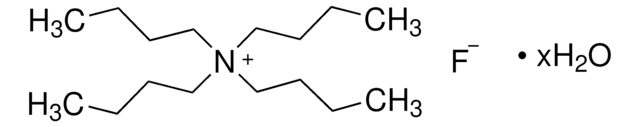
Tetrabutylammonium fluoride trihydrate
≥97.0% (NT)
Sinónimos:
TBAF
About This Item
Productos recomendados
Quality Level
assay
≥97.0% (NT)
form
crystals
mp
62-63 °C (lit.)
SMILES string
O.O.O.[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH.3H2O/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;;;;/h5-16H2,1-4H3;1H;3*1H2/q+1;;;;/p-1
InChI key
VEPTXBCIDSFGBF-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
- For the dehydrobromination of vinyl bromides to terminal acetylenes.
- In the conversion of 1,1-dibromo-1-alkenes to terminal alkynes via Corey–Fuchs reaction.
- In Hiyama cross-coupling reaction of aryl and heteroaryl chlorides with aryltrialkoxysilanes in the presence of a palladium catalyst.
It can be used to catalyze ethynylation of quinolines and isoquinolines using calcium carbide in aqueous N,N-dimethylacetamide.
Other Notes
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico