762024
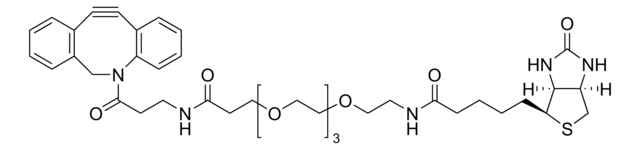
Azide-PEG3-biotin conjugate
Sinónimos:
Polyethylene glycol, N-[2-[2-[2-(2-Azidoethoxy)ethoxy]ethoxy]ethyl]hexahydro-2-oxo-(3aS,4S,6aR)- 1H-thieno[3,4-d ]imidazole-4-pentanamide, Biotin-PEG3-azide
About This Item
Productos recomendados
form
solid
Quality Level
reaction suitability
reaction type: click chemistry
storage temp.
−20°C
SMILES string
O=C1N[C@](CS[C@H]2CCCCC(NCCOCCOCCOCCN=[N+]=[N-])=O)([H])[C@]2([H])N1
InChI
1S/C18H32N6O5S/c19-24-21-6-8-28-10-12-29-11-9-27-7-5-20-16(25)4-2-1-3-15-17-14(13-30-15)22-18(26)23-17/h14-15,17H,1-13H2,(H,20,25)(H2,22,23,26)/t14-,15-,17-/m1/s1
InChI key
ZWFOOMQCYIGZBE-BFYDXBDKSA-N
Application
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Contenido relacionado
Polyethylene glycol (PEG), also sometimes referred to as polyethylene oxide (PEO), is a condensation polymer of ethylene oxide and water that has several chemical properties that make it useful for biological, chemical and pharmaceutical applications.
Polyethylene glycol (PEG), also sometimes referred to as polyethylene oxide (PEO), is a condensation polymer of ethylene oxide and water that has several chemical properties that make it useful for biological, chemical and pharmaceutical applications.
Polyethylene glycol (PEG), also sometimes referred to as polyethylene oxide (PEO), is a condensation polymer of ethylene oxide and water that has several chemical properties that make it useful for biological, chemical and pharmaceutical applications.
Polyethylene glycol (PEG), also sometimes referred to as polyethylene oxide (PEO), is a condensation polymer of ethylene oxide and water that has several chemical properties that make it useful for biological, chemical and pharmaceutical applications.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)