419214
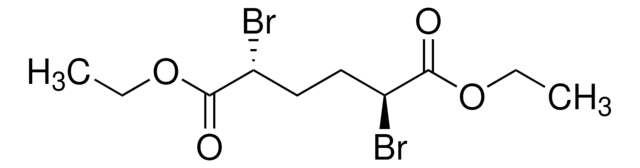
Dimethyl 2,6-dibromoheptanedioate
97%
Sinónimos:
2,6-Dibromoheptanedioic acid dimethyl ester
About This Item
Productos recomendados
assay
97%
form
liquid
greener alternative product score
old score: 2
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Safer Solvents and Auxiliaries
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.501 (lit.)
bp
130-140 °C/0.01 mmHg (lit.)
density
1.59 g/mL at 25 °C (lit.)
greener alternative category
SMILES string
COC(=O)C(Br)CCCC(Br)C(=O)OC
InChI
1S/C9H14Br2O4/c1-14-8(12)6(10)4-3-5-7(11)9(13)15-2/h6-7H,3-5H2,1-2H3
Inchi Key
AWWJYEJSCIDADZ-UHFFFAOYSA-N
General description
Application
- Preparation of difunctional poly(n-butyl acrylate) (pBA) macroinitiator.
- As initiator for the synthesis of dibromo-terminated polystyrene, via atom transfer radical polymerization (ATRP).
- Preparation of polytrithiocarbonate, which serves as Reversible Addition-Fragmentation chain Transfer (RAFT) agent for radical polymerization reactions.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico



![Tris[2-(dimethylamino)ethyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/695/792/ee0ff167-22a3-43a7-83a1-6c4908adf0ae/640/ee0ff167-22a3-43a7-83a1-6c4908adf0ae.png)





![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol](/deepweb/assets/sigmaaldrich/product/structures/839/520/64c23004-f340-460f-a379-8670a35d0433/640/64c23004-f340-460f-a379-8670a35d0433.png)


