244112
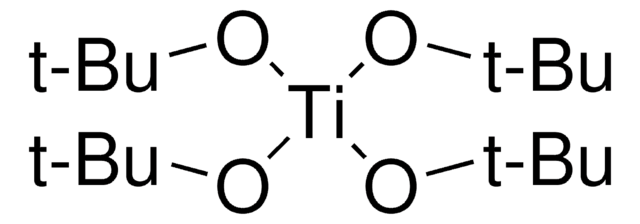
Butóxido de titanio (IV)
reagent grade, 97%
Sinónimos:
Ortotitanato de tetrabutilo, TNBT, Titanato de tetrabutilo, Titanato orgánico TYZOR® TBT, Ácido ortotitánico tetrabutiléster
About This Item
Productos recomendados
grade
reagent grade
assay
97%
form
liquid (or viscous liquid)
liquid
reaction suitability
core: titanium
reagent type: catalyst
refractive index
n20/D 1.491 (lit.)
bp
206 °C/10 mmHg (lit.)
density
1.00 g/mL at 20 °C (lit.)
SMILES string
CCCCO[Ti](OCCCC)(OCCCC)OCCCC
InChI
1S/4C4H9O.Ti/c4*1-2-3-4-5;/h4*2-4H2,1H3;/q4*-1;+4
InChI key
YHWCPXVTRSHPNY-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Central nervous system, Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
107.6 °F - Pensky-Martens closed cup
flash_point_c
42 °C - Pensky-Martens closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Protocolos
Polymeric spheres serve as crystal templates. Synthesis methods yield large quantities.
Polymeric spheres serve as crystal templates. Synthesis methods yield large quantities.
Polymeric spheres serve as crystal templates. Synthesis methods yield large quantities.
Polymeric spheres serve as crystal templates. Synthesis methods yield large quantities.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico