All Photos(2)
About This Item
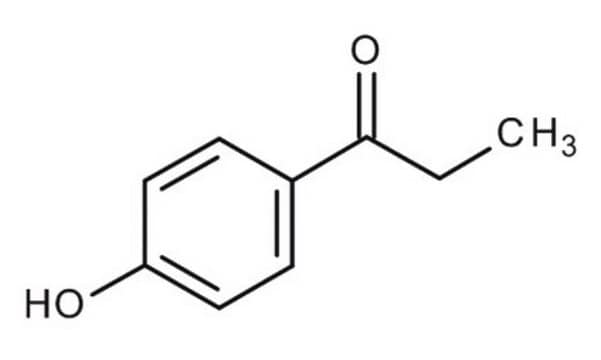
Linear Formula:
HOC6H4COC2H5
CAS Number:
Molecular Weight:
150.17
Beilstein:
907511
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
147.5-148.5 °C (lit.)
SMILES string
CCC(=O)c1ccc(O)cc1
InChI
1S/C9H10O2/c1-2-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
InChI key
RARSHUDCJQSEFJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Cizmáriková et al.
Ceska a Slovenska farmacie : casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti, 43(5), 226-228 (1994-10-01)
The present paper carries out the pharmacological evaluation of 4-(2-hydroxy-3-isopropylaminopropoxy)-3-(alkoxymethyl) propiophenones with an ethoxy, propoxy and butoxy-group, whose structures are typical of the blockers of beta-adrenergic receptors. In the above-mentioned compounds the anticalcium effect on the frequency and the amplitude
R Cizmáriková et al.
Ceskoslovenska farmacie, 39(9), 403-408 (1990-11-01)
Within the framework of the study of the structure-effect relationship, a series of novel potential beta-adrenolytic agents derived from p-hydroxyacetophenone and p-hydroxypropiophenone with an alyloxymethyl and a cycloalkyloxymethyl group in the lipophilic part of the molecule and an isopropyl and
p-Hydroxypropiophenone effects on azo dye-induced alterations in mouse hepatic cells: light and electron microscopic study.
N J Unakar
Journal of the National Cancer Institute, 44(4), 873-891 (1970-04-01)
A Tanner et al.
Journal of bacteriology, 182(23), 6565-6569 (2000-11-14)
An arylketone monooxygenase was purified from Pseudomonas putida JD1 by ion exchange and affinity chromatography. It had the characteristics of a Baeyer-Villiger-type monooxygenase and converted its substrate, 4-hydroxyacetophenone, into 4-hydroxyphenyl acetate with the consumption of one molecule of oxygen and
F da S Knudsen et al.
Photochemistry and photobiology, 55(2), 267-277 (1992-02-01)
In the presence of the surfactant hexadecyltrimethyl ammonium bromide (CTAB) a cascade of electronically excited states accompanies the successive steps in the peroxidative metabolization of the strong estrogenic and tumourogenic diethylstilbestrol. Reversing the order by necessity, we report in this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service