All Photos(3)
About This Item
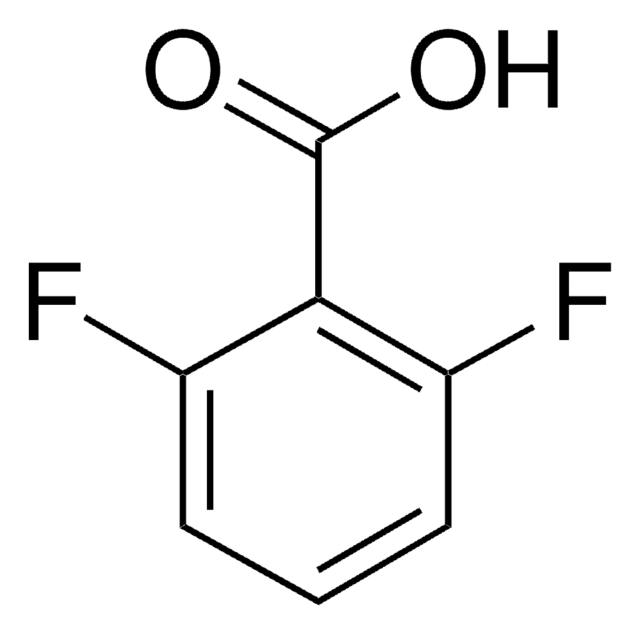
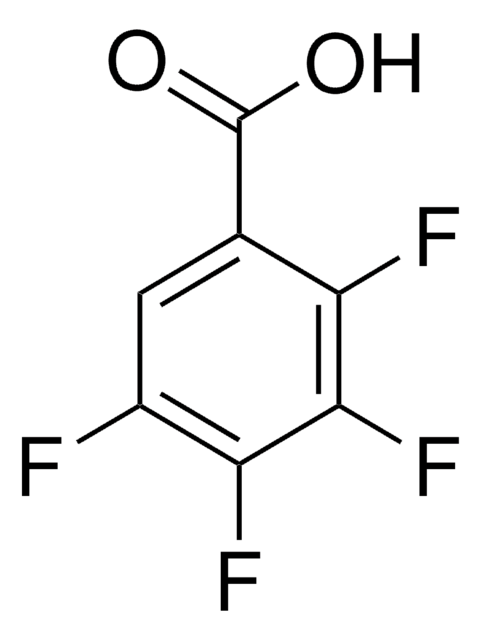
Linear Formula:
FC6H4CO2H
CAS Number:
Molecular Weight:
140.11
Beilstein:
1906920
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
122-124 °C (lit.)
SMILES string
OC(=O)c1cccc(F)c1
InChI
1S/C7H5FO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,(H,9,10)
InChI key
MXNBDFWNYRNIBH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F G Hidde Boersma et al.
FEMS microbiology letters, 237(2), 355-361 (2004-08-24)
While several microorganisms readily degrade 2- and 4-fluorobenzoates, only a very small number appear to catabolise the 3-fluoro isomer, owing to the accumulation of toxic intermediates. Here we describe the isolation of a bacterium capable of using 3-fluorobenzoate as a
J B J H van Duuren et al.
Journal of biotechnology, 156(3), 163-172 (2011-09-13)
Pseudomonas putida KT2440-JD1 was derived from P. putida KT2440 after N-methyl-N'-nitro-N-nitrosoguanidine (NTG)-mutagenesis and exposure to 3-fluorobenzoate (3-FB). The mutant was no longer able to grow using benzoate as a sole carbon source, but co-metabolized benzoate to cis, cis-muconate during growth
C J Springer et al.
Journal of medicinal chemistry, 37(15), 2361-2370 (1994-07-22)
The synthesis of six novel fluorinated potential prodrugs for antibody-directed enzyme prodrug therapy is described. The [2- and 3-fluoro-4-[bis(2-chloroethyl)amino]benzoyl]-L-glutamic acid (9 and 21), their bis(mesyloxy)ethyl derivatives (7 and 19), and their chloroethyl (mesyloxy)-ethyl derivatives (8 and 20) are bifunctional alkylating
Metabolism of monofluoro- and monochlorobenzoates by a dentrifying bacterium.
B F Taylor et al.
Archives of microbiology, 122(3), 301-306 (1979-09-01)
R Miura
Journal of biochemistry, 105(2), 318-322 (1989-02-01)
The interactions of competitive inhibitors, o-, m-, and p-fluorobenzoates, with porcine kidney D-amino acid oxidase (DAO) were studied by 19F-NMR spectroscopy. The 19F-signals of DAO-bound fluorobenzoates were observed as considerably broadened peaks. The chemical shifts, which are referenced to 20
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service