327972
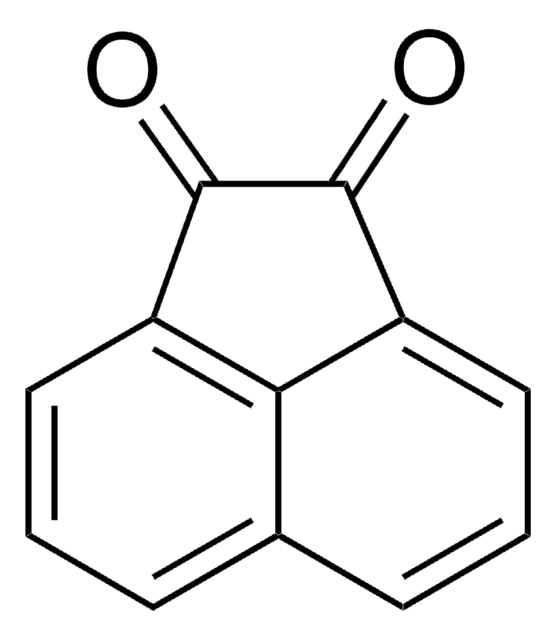
Aceanthrenequinone
96%
Synonym(s):
1,2-Aceanthrylenedione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C16H8O2
CAS Number:
Molecular Weight:
232.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
270-273 °C (lit.)
SMILES string
O=C1C(=O)c2c3ccccc3cc4cccc1c24
InChI
1S/C16H8O2/c17-15-12-7-3-5-10-8-9-4-1-2-6-11(9)14(13(10)12)16(15)18/h1-8H
InChI key
YAIBDWAANBTYIA-UHFFFAOYSA-N
General description
Aceanthrenequinone is a cyclic α-diketone. It reacts with hexaethyltriaminophosphine in the presence of fullerene C(60), to yield methanofullerene derivatives. Hydroxyalkylation reactions of aceanthrenequinone with a series of arenes was reported.
Application
Aceanthrenequinone was used in synthesis of spiro-tricyclic porphodimethenes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Harmjanz et al.
Organic letters, 3(15), 2281-2284 (2001-07-21)
[structure: see text] Acid-catalyzed [2 + 2] condensation reactions of polycyclic aromatic vicinal diketones including aceanthrenequinone, phenathrenequinone, and pyrene-4,5-dione with 5-mesityldipyrromethanes are outlined, and this methodology provides a flexible entry to spiro-tricyclic porphodimethenes. The porphodimethene products have been fully characterized
Douglas A Klumpp et al.
Applied catalysis. A, General, 336(1-2), 128-132 (2008-03-01)
The hydroxyalkylation reactions of aceanthrenequinone (6) and acenapthenequinone (7) with a series of arenes have been studied. In reactions with the Brønsted superacid CF(3)SO(3)H (triflic acid), the condensation products are formed in good yields (58-99%, 10 examples) with high regioselectivity.
Irina P Romanova et al.
The Journal of organic chemistry, 76(8), 2548-2557 (2011-03-12)
The reactions of such cyclic α-diketones as acenaphthenequinone, aceanthrenequinone, and N-alkylisatins, with hexaethyltriaminophosphine in the presence of the fullerene C(60), lead to the formation of methanofullerene derivatives under mild conditions. This process proceeds via deoxygenation of the dicarbonyl compound by
Janice L Hyatt et al.
Journal of medicinal chemistry, 50(23), 5727-5734 (2007-10-19)
Carboxylesterases (CE) are ubiquitous enzymes responsible for the detoxification of xenobiotics, including numerous clinically used drugs. Therefore, the selective inhibition of these proteins may prove useful in modulating drug half-life and bioavailability. Recently, we identified 1,2-diones as potent inhibitors of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service