190438
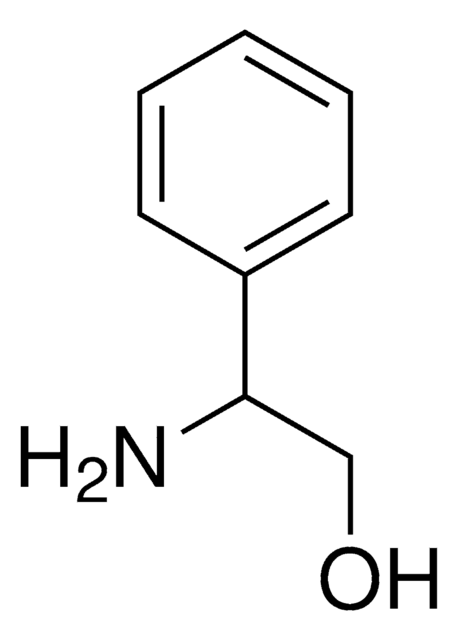
(S)-(−)-2-Amino-3-phenyl-1-propanol
98%, optical purity ee: 99% (HPLC)
Synonym(s):
L-Phenylalaninol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH(NH2)CH2OH
CAS Number:
Molecular Weight:
151.21
Beilstein:
2208238
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
optical activity
[α]22/D −22.8°, c = 1.2 in 1 M HCl
optical purity
ee: 99% (HPLC)
mp
92-94 °C (lit.)
SMILES string
N[C@H](CO)Cc1ccccc1
InChI
1S/C9H13NO/c10-9(7-11)6-8-4-2-1-3-5-8/h1-5,9,11H,6-7,10H2/t9-/m0/s1
InChI key
STVVMTBJNDTZBF-VIFPVBQESA-N
Related Categories
General description
(S)-(-)-2-Amino-3-phenyl-1-propanol is a chiral amino alcohol.
Application
(S)-(-)-2-Amino-3-phenyl-1-propanol can undergo condensation with 5-nitrosalicylaldehyde to form (S)-2-[(1-benzyl-2-hydroxyethylimino)methyl]-4-nitrophenol, a new chiral Schiff base. It reacts with substituted salicylaldehydes to form tridentate chiral Schiff base ligands, which can form H-bonded chiral supramolecular metal-organic architectures. It can also be used in the synthesis of an unnatural tripeptide, which can enhance the antimicrobial activity of methicillin against methicillin resistant Staphylococcus aureus.
Reacts with nitriles to form oxazolines which are useful in Pd-catalyzed allylic substitution. Also employed in amidation for chiral resolution and NADH modeling.
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tetrahedron, 49, 5237-5237 (1993)
Tripeptide LY301621 and its diastereomers as methicillin potentiators against methicillin resistant Staphylococcus aureus.
Bid CN, et al.
The Journal of Antibiotics, 50(3), 283-285 (1997)
G P Zecchini et al.
Archiv der Pharmazie, 328(9), 673-676 (1995-09-01)
The synthesis and the biological activity towards human neutrophils of some N-formyl-Met-Leu-Phe-OMe analogues containing (S)-phenylalaninol (Pheol) or its derivatives in place of the native phenylalanine are reported. While the analogue containing Pheol (4) was found to be devoid of significant
B Weiss et al.
Research communications in chemical pathology and pharmacology, 62(1), 113-123 (1988-10-01)
An amino acid derivative, leucinethiol, was reported to be a strong inhibitor of aminopeptidase activity. In order to obtain selective inhibitors of various brain aminopeptidases, we tested the inhibition by amino acid analogs of brain aminopeptidase activity. In particular, we
Violetta Constantinou-Kokotou et al.
Journal of peptide science : an official publication of the European Peptide Society, 11(7), 431-435 (2005-01-07)
2-Oxoamides based on long chain beta-amino acids were synthesized. 1-Benzyl substituted long chain amines, needed for such synthesis, were synthesized starting from Boc-phenylalaninol. The oxidative conversion of a phenyl group to a carboxyl group was used as the key transformation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service