04473
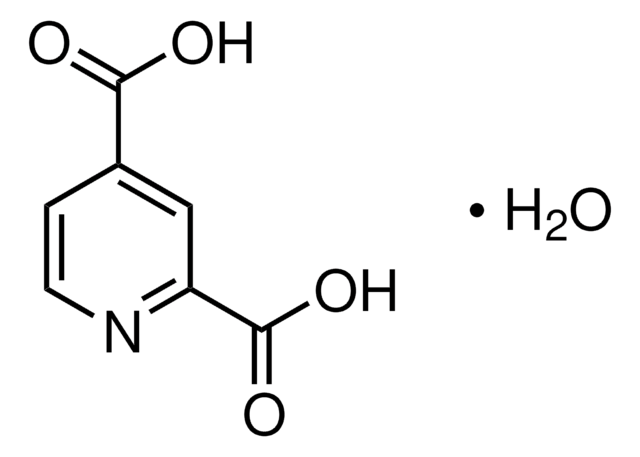
2,4-Pyridinedicarboxylic acid
≥98.0%
Synonym(s):
Lutidinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5NO4
CAS Number:
Molecular Weight:
167.12
Beilstein:
131631
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥98.0%
98.0-102.0% (T)
SMILES string
OC(=O)c1ccnc(c1)C(O)=O
InChI
1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
InChI key
MJIVRKPEXXHNJT-UHFFFAOYSA-N
Application
2,4-Pyridinedicarboxylic acid is an in vitro and in cell inhibitor, as well as a known inhibitor of the histone lysine demethylases. 2,4-Pyridinedicarboxylic acid has been used in a study to determine that ruthenium(II) complexes exert antimetastatic effects on several tumor cell lines in vitro, achieved mostly by the effect on cell adhesion, migration and angiogenesis. . 2,4-Pyridinedicarboxylic acid has been used in a study to develop an assay that represents the first report of a RapidFire mass spectrometery assay for an epigenetics target.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nevenka Gligorijević et al.
Journal of inorganic biochemistry, 108, 53-61 (2012-01-24)
In our previous study, ruthenium(II)-p-cymene complexes of general formula [(η(6)-p-cymene)Ru(L)Cl2], L: 3-acetylpyridine (1), 2-amino-5-chloropyridine (2); and [(η(6)-p-cymene)Ru(HL)Cl], HL: 2,3-pyridinedicarboxylic acid (3), 2,4-pyridinedicarboxylic acid (4), revealed low antiproliferative activity, except complex [(η(6)-p-cymene)RuCl(picolinic acid)]·H(2)O (5) which exhibited IC(50) around 80 μM. In
Gunnar Schley et al.
The American journal of pathology, 181(5), 1595-1606 (2012-09-05)
The role of proximal versus distal tubular injury in the pathogenesis of acute kidney injury (AKI) is debatable. Inhibition of prolyl hydroxylases that regulate the degradation of hypoxia-inducible transcription factors (HIFs) is a promising therapeutic approach to optimize energy preservation
H M Hanauske-Abel
Journal of hepatology, 13 Suppl 3, S8-15 (1991-01-01)
The hydrophilic compound pyridine 2,4-dicarboxylate (2,4-PDCA), designed as a mechanism-based competitive inhibitor of prolyl 4-hydroxylase, is efficiently excluded by the cytoplasmic membrane, but permeates the endoplasmic membrane via a 2,4-PDCA-selective translocator to reach its target enzyme in the intracisternal space.
C K Derian et al.
The Journal of biological chemistry, 264(12), 6615-6618 (1989-04-25)
While a role has been ascribed to the gamma-carboxyglutamate (Gla) residues in vitamin K-dependent coagulation proteins and the enzyme catalyzing this posttranslational modification has been identified and partially characterized, both the functional significance of a second posttranslationally synthesized amino acid
Line H Kristensen et al.
The FEBS journal, 279(11), 1905-1914 (2012-03-17)
Dynamic methylations and demethylations of histone lysine residues are important for gene regulation and are facilitated by histone methyltransferases and histone demethylases (HDMs). KDM5B/Jarid1B/PLU1 is an H3K4me3/me2-specific lysine demethylase belonging to the JmjC domain-containing family of histone demethylases (JHDMs). Several
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service