All Photos(1)
About This Item
Empirical Formula (Hill Notation):
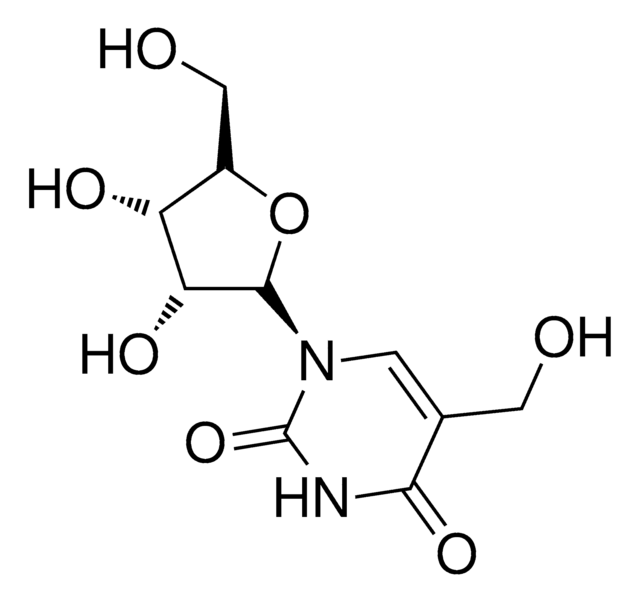
C5H6N2O3
CAS Number:
Molecular Weight:
142.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
>300 °C (lit.)
functional group
hydroxyl
SMILES string
[H]O[H].OCC1=CNC(=O)NC1=O
InChI
1S/C5H6N2O3/c8-2-3-1-6-5(10)7-4(3)9/h1,8H,2H2,(H2,6,7,9,10)
InChI key
JDBGXEHEIRGOBU-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Masaki Hori et al.
Nucleic acids research, 31(4), 1191-1196 (2003-02-13)
The oxidation and deamination of 5-methylcytosine (5mC) in DNA generates a base-pair between 5-hydroxymethyluracil (5hmU) and guanine. 5hmU normally forms a base-pair with adenine. Therefore, the conversion of 5mC to 5hmU is a potential pathway for the generation of 5mC
Hideharu Hashimoto et al.
Nucleic acids research, 40(11), 4841-4849 (2012-03-01)
Cytosine residues in mammalian DNA occur in at least three forms, cytosine (C), 5-methylcytosine (M; 5mC) and 5-hydroxymethylcytosine (H; 5hmC). During semi-conservative DNA replication, hemi-methylated (M/C) and hemi-hydroxymethylated (H/C) CpG dinucleotides are transiently generated, where only the parental strand is
Rafal Rozalski et al.
Free radical research, 38(11), 1201-1205 (2004-12-29)
In order to eliminate the possibility that diet may influence urinary oxidative DNA lesion levels, in our experiments we used a recently developed technique involving HPLC pre-purification followed by gas chromatography with isotope dilution mass spectrometric detection. This methodology was
Hideharu Hashimoto et al.
Nucleic acids research, 40(17), 8276-8284 (2012-06-29)
The mammalian DNA glycosylase--methyl-CpG binding domain protein 4 (MBD4)--is involved in active DNA demethylation via the base excision repair pathway. MBD4 contains an N-terminal MBD and a C-terminal DNA glycosylase domain. MBD4 can excise the mismatched base paired with a
Neeraj Shakya et al.
Bioorganic & medicinal chemistry, 20(13), 4088-4097 (2012-06-06)
Discovery of novel antimycobacterial compounds that work on distinctive targets and by diverse mechanisms of action is urgently required for the treatment of mycobacterial infections due to the emerging global health threat of tuberculosis. We have identified a new class
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service