All Photos(2)
About This Item
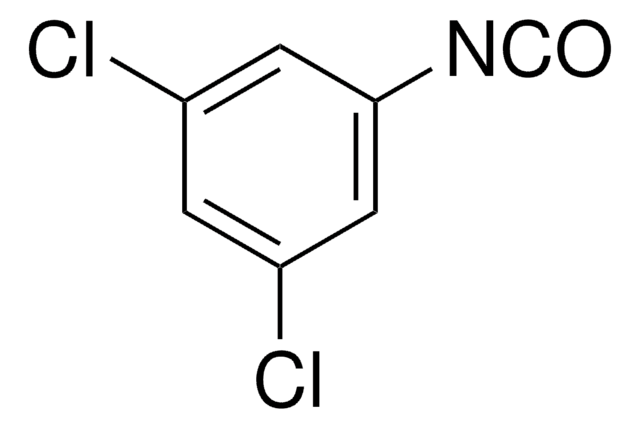
Linear Formula:
NCC6H4NCO
CAS Number:
Molecular Weight:
144.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
51-54 °C (lit.)
functional group
isocyanate
nitrile
SMILES string
O=C=Nc1cccc(c1)C#N
InChI
1S/C8H4N2O/c9-5-7-2-1-3-8(4-7)10-6-11/h1-4H
InChI key
NZHPVPMRNASEQK-UHFFFAOYSA-N
General description
3-Cyanophenyl isocyanate is an aromatic isocyanate.
Application
3-Cyanophenyl isocyanate may be employed as starting reagent for the following syntheses:
- R/S-4-(3-chlorophenylaminocarbonylamino)-3,4-dihydro-2,2-dimethyl-2H-1-benzopyran
- 1-(3-cyanophenyl)-3-(2-methoxy-6-pentadecylbenzyl)urea
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and Antibacterial Activity of Urea and hiourea Derivatives at C-8 Alkyl Chain of Anacardic Acid Mixture Isolated from a Natural Product Cashew Nut Shell Liquid (CNSL).
Reddy NS, et al.
International Journal of Organic Chemistry, 1(04), 167-167 (2011)
Smail Khelili et al.
Bioorganic & medicinal chemistry, 16(11), 6124-6130 (2008-05-16)
Ring-opened analogues of dihydrobenzopyran potassium channel openers (PCOs) were prepared and evaluated as putative PCOs on rat aorta rings (myorelaxant effect) and rat pancreatic beta-cells (inhibition of insulin secretion). These derivatives are characterized by the presence of a sulfonylurea, a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service