424471
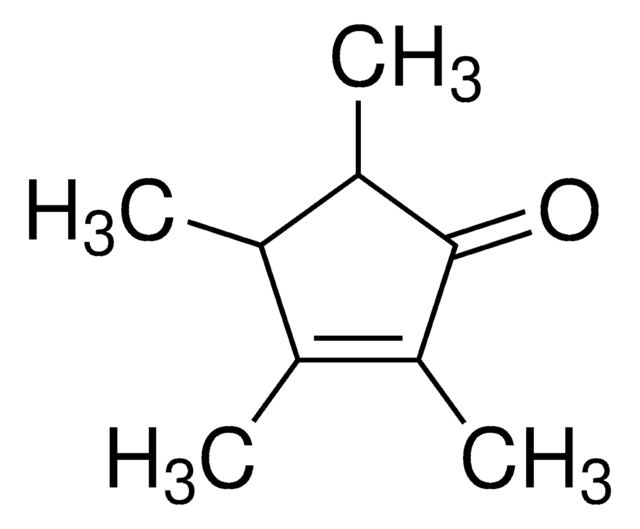
1,2,3,4-Tetramethyl-1,3-cyclopentadiene
~85%
Synonym(s):
1,2,3,4-Tetramethylcyclopentadiene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H14
CAS Number:
Molecular Weight:
122.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
~85%
form
liquid
refractive index
n20/D 1.472 (lit.)
bp
142 °C (lit.)
density
0.808 g/mL at 25 °C (lit.)
SMILES string
CC1=C(C)C(C)=C(C)C1
InChI
1S/C9H14/c1-6-5-7(2)9(4)8(6)3/h5H2,1-4H3
InChI key
VNPQQEYMXYCAEZ-UHFFFAOYSA-N
General description
1,2,3,4-Tetramethyl-1,3-cyclopentadiene is a tetra substituted 1,3-cyclopentadiene. It participates in the synthesis of resin-bound tetramethylcyclopentadienes.
Application
1,2,3,4-Tetramethyl-1,3-cyclopentadiene may be used as a starting material in the synthesis of 1,7,8,9-tetramethyl-4-oxa-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione.
Other Notes
remainder mixture of isomers
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The synthesis of resin-bound tetramethylcyclopentadienes: an evaluation of two methodologies.
Shearer AS and de Miguel YR.
Tetrahedron Letters, 47(4), 447-450 (2006)
Selvan Demir et al.
Nature communications, 8(1), 2144-2144 (2017-12-17)
Increasing the operating temperatures of single-molecule magnets-molecules that can retain magnetic polarization in the absence of an applied field-has potential implications toward information storage and computing, and may also inform the development of new bulk magnets. Progress toward these goals
Synthesis of 4-Amino-1, 7, 8, 9-tetramethyl-4-aza-tricyclo [5.2. 1.02,6] dec-8-ene-3, 5-dione.
Struga M and Kossakowski J.
Molbank, 2007(2), M534-M534 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service