All Photos(1)
About This Item
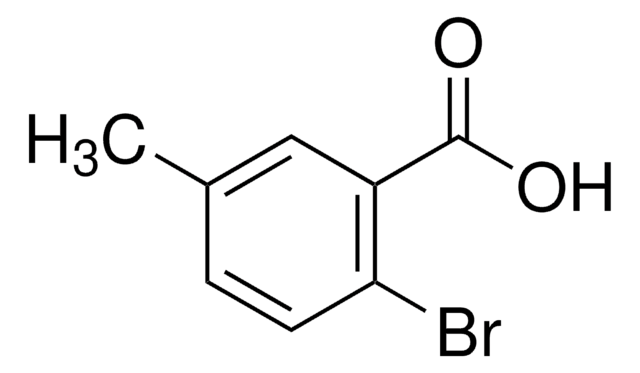
Linear Formula:
BrC6H3(OCH3)CO2H
CAS Number:
Molecular Weight:
231.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
157-159 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
COc1ccc(Br)c(c1)C(O)=O
InChI
1S/C8H7BrO3/c1-12-5-2-3-7(9)6(4-5)8(10)11/h2-4H,1H3,(H,10,11)
InChI key
ODHJOROUCITYNF-UHFFFAOYSA-N
Related Categories
General description
2-Bromo-5-methoxybenzoic acid is a benzoic acid derivative. Synthesis of 2-bromo-5-methoxybenzoic acid and its characterization by HNMR has been reported.
Application
2-Bromo-5-methoxybenzoic acid is suitable for use in the syntheses of urolithin derivatives. It may be used in the synthesis of the following:
- substituted aminobenzacridines
- 8-chloro-2-methoxydibenzo[b,f]thiepin-10(11H)-one and its 3-methoxy derivative
- isoindolinone derivatives
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Amirhossein Fallah et al.
Journal of fluorescence, 30(1), 113-120 (2020-01-04)
The detection and sensing of environmentally crucial metal ions has always been of great significance in various fields such as biological and environmental cycles. Our previous studies have indicated a new coumarin based lactone, Urolithin B (i.e., 3-Hydroxy[c]chromen-6-one) as a
Potential metabolites of clorotepin: 2-and 3-hydroxy derivatives of 8-chloro-10-(4-methylpiperazino)-10, 11-dihydrodibenzo [b, f] thiepin and their methyl ethers.
Sindelar, K., et al.
Collection of Czechoslovak Chemical Communications, 39(12), 3548-3559 (1974)
Lynn S Adams et al.
Cancer prevention research (Philadelphia, Pa.), 3(1), 108-113 (2010-01-07)
Estrogen stimulates the proliferation of breast cancer cells and the growth of estrogen-responsive tumors. The aromatase enzyme, which converts androgen to estrogen, plays a key role in breast carcinogenesis. The pomegranate fruit, a rich source of ellagitannins (ET), has attracted
Design, synthesis and biological evaluation of the novel isoindolinone derivatives.
Liu J, et al.
J. Chem. Res. (M), 6(9), 256-260 (2014)
Synthesis of substituted aminobenzacridines.
G B BACHMAN et al.
Journal of the American Chemical Society, 68, 1599-1602 (1946-08-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)