324906
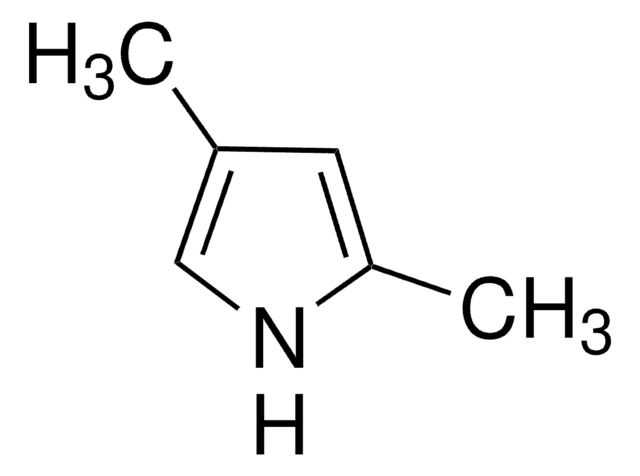
4,5,6,7-Tetrahydroindole
98%
Synonym(s):
2,3-Tetramethylenepyrrole, Cyclohex[b]pyrrole, NSC 122455
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H11N
CAS Number:
Molecular Weight:
121.18
Beilstein:
108853
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
53-57 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CCc2[nH]ccc2C1
InChI
1S/C8H11N/c1-2-4-8-7(3-1)5-6-9-8/h5-6,9H,1-4H2
InChI key
KQBVVLOYXDVATK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4,5,6,7-Tetrahydroindoles, due to their easy aromatization, are good intermediates to synthesize indoles. 4,5,6,7-Tetrahydroindole on condensation with cyanoacetate leads to 1-ethylthio-2-cyano-4,5,6,7-tetrahydrocyclohexa-[c]-3H-pyrrolizin-3-one.
Application
4,5,6,7-Tetrahydroindole was used as reactant in:
- synthesis of ethyl 3-(4,5,6,7-tetrahydroindol-2-yl)-2-propynoate
- preparation of BODIPY dyes
- N-alkylation with chloromethyloxirane
- preparation of hydroindolepropynoate by chemo- and regioselective solvent-free ethynylation
- palladium- and copper-free cross-coupling of halopropynoates
- preparation of carbonylalkenyl indoles via coupling with dicarbonyl compounds
- 1:2 annelation of 4,5,6,7-tetrahydroindole with 1-benzoyl-2-phenylacetylene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Markova, M., V.; et al.
ARKIVOC (Gainesville, FL, United States), 57-57 (2008)
Arcadi, A.; et al.
Advanced Synthesis & Catalysis, 348, 331-331 (2006)
Pyrrole-2-dithiocarboxylates: Synthesis of 2-(1-Alkylthio-2-cyanoethenyl) pyrroles.
Sobenina LN, et al.
Tetrahedron, 51(14), 4223-4230 (1995)
Trofimov, B. A.; et al.
Tetrahedron Letters, 48, 4661-4661 (2007)
Volker Leen et al.
Chemical communications (Cambridge, England), (30), 4515-4517 (2009-07-21)
A careful choice of the pyrrole building blocks allows the synthesis of a wide range of monohalogenated BODIPY dyes with excellent reactivity in palladium catalyzed coupling reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service