324663
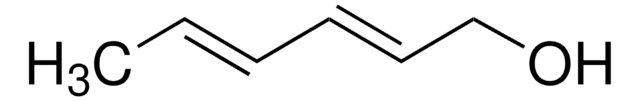
1,4-Pentadien-3-ol
contains 0.4% hydroquinone as stabilizer, ≥96%
Synonym(s):
Divinyl carbinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH(OH)CH=CH2
CAS Number:
Molecular Weight:
84.12
Beilstein:
1735809
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥96%
form
liquid
contains
0.4% hydroquinone as stabilizer
refractive index
n20/D 1.445 (lit.)
bp
115-116 °C (lit.)
density
0.865 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OC(C=C)C=C
InChI
1S/C5H8O/c1-3-5(6)4-2/h3-6H,1-2H2
InChI key
ICMWSAALRSINTC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Starting material for asymmetric epoxidation.
Substrate employed in a synthesis of amino-substituted dienes via a bismuth-catalyzed SNi displacement of alcohols by sulfonamide nucleophiles.
Useful building block in natural product synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
89.6 °F - closed cup
Flash Point(C)
32 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Masanori Imai et al.
The Journal of organic chemistry, 69(4), 1144-1150 (2004-02-14)
Intermolecular hydroacylation between salicylaldehydes 1, 26-40 and 1,4-penta- or 1,5-hexadienes 4-13 by Rh-catalyst proceeded under mild reaction conditions to give a mixture of iso- and normal-hydroacylated products 14-25, 41-55, and 57-60. In the hydroacylation reaction, chelation of both salicylaldehyde and
Tetrahedron Asymmetry, 4, 1533-1533 (1993)
Chin. J. Chem., 9, 381-381 (1991)
P Andrew Evans et al.
Journal of the American Chemical Society, 125(48), 14702-14703 (2003-12-04)
The enantioselective total synthesis of the annonaceous acetogenin (-)-mucocin (1) was accomplished using a triply convergent 12-step sequence (longest linear sequence) in 13.6% overall yield. This represents the first application of the temporary silicon-tethered (TST) ring-closing metathesis (RCM) cross-coupling reaction
Bismuth-catalyzed direct substitution of the hydroxy group in alcohols with sulfonamides, carbamates, and carboxamides.
Hongbo Qin et al.
Angewandte Chemie (International ed. in English), 46(3), 409-413 (2006-12-06)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service