29524
(R)-(6,6′-Dimethoxybiphenyl-2,2′-diyl)bis[bis(3,5-di-tert-butylphenyl)phosphine]
≥97%, optical purity ee: ≥99%
Synonym(s):
(R)-2,2′-Bis[bis(3,5-di-tert-butyl)phosphino]-6,6′-dimethoxy-1,1′-biphenyl, (R)-3,5-t-Bu-MeOBIPHEP, SL-A121-1
About This Item
Recommended Products
Assay
≥97%
optical purity
ee: ≥99%
functional group
phosphine
SMILES string
COc1cccc(P(c2cc(cc(c2)C(C)(C)C)C(C)(C)C)c3cc(cc(c3)C(C)(C)C)C(C)(C)C)c1-c4c(OC)cccc4P(c5cc(cc(c5)C(C)(C)C)C(C)(C)C)c6cc(cc(c6)C(C)(C)C)C(C)(C)C
InChI
1S/C70H96O2P2/c1-63(2,3)45-33-46(64(4,5)6)38-53(37-45)73(54-39-47(65(7,8)9)34-48(40-54)66(10,11)12)59-31-27-29-57(71-25)61(59)62-58(72-26)30-28-32-60(62)74(55-41-49(67(13,14)15)35-50(42-55)68(16,17)18)56-43-51(69(19,20)21)36-52(44-56)70(22,23)24/h27-44H,1-26H3
InChI key
PBYRAYONARLAQJ-UHFFFAOYSA-N
General description
Application
- 1,2-Dihydropyridines through Rh-catalyzed cycloaddition of diynes to sulfonimines.
- Enantiorich disubstituted γ-lactams via intramolecular allylic alkylation reaction using Pd catalyst.
- A Ru-metal complex, which acts as a hydrogenation catalyst applicable in the synthesis of an organic building block (S)-3-fluoromethyl-γ-butyrolactone.
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Solvias MeOBIPHEP Ligands: State-of-the-art atropisomeric MeOBIPHEP ligands, also referred to as MeO-BIPHEP, originally developed by Roche, have an extraordinarily broad performance profile for many synthetic applications due to their modular ligand design.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service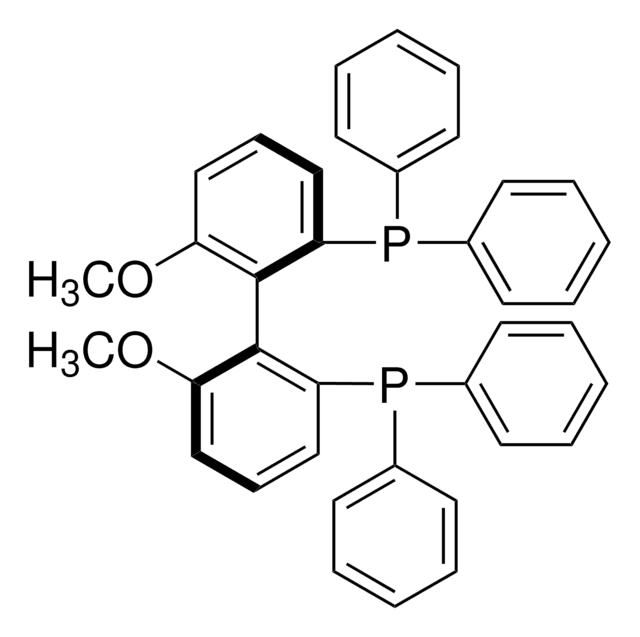
![(S)-(6,6′-Dimethoxybiphenyl-2,2′-diyl)bis[bis(3,5-dimethylphenyl)phosphine] ≥97%, optical purity ee: ≥99%](/deepweb/assets/sigmaaldrich/product/structures/178/629/39b4bd16-5ab1-4830-8f97-5b030b659e11/640/39b4bd16-5ab1-4830-8f97-5b030b659e11.png)
![(R)-(6,6′-Dimethoxybiphenyl-2,2′-diyl)bis[bis(3,5-dimethylphenyl)phosphine] ≥97% (31P-NMR), optical purity ee: ≥99%](/deepweb/assets/sigmaaldrich/product/structures/377/820/32e028a2-0c7d-460d-8b6f-668b6d6a1523/640/32e028a2-0c7d-460d-8b6f-668b6d6a1523.png)





![Dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/351/904/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3/640/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3.png)
