195944
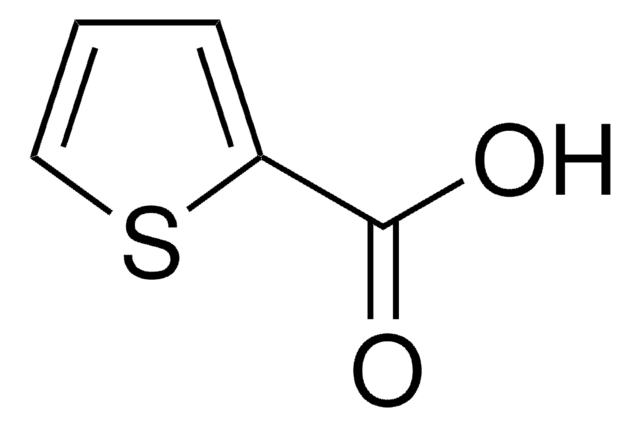
2-Thiopheneacetic acid
98%
Synonym(s):
2-Thienylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H6O2S
CAS Number:
Molecular Weight:
142.18
Beilstein:
114551
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
160 °C/22 mmHg (lit.)
mp
63-64 °C (lit.)
SMILES string
OC(=O)Cc1cccs1
InChI
1S/C6H6O2S/c7-6(8)4-5-2-1-3-9-5/h1-3H,4H2,(H,7,8)
InChI key
SMJRBWINMFUUDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Adsorption of 2-thiopheneacetic acid on XAD-4, NDA-100 and ND-90 resin has been investigated. A new polymeric complex of Cu(II) and 2-thiopheneacetic acid has been synthesized and characterized by IR and Raman spectroscopy.
Application
2-Thiopheneacetic acid was used in the preparation of rosette-like nanoscale Au materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, X-ray crystal structure and vibrational spectra of a polymeric copper (II) complex with 2-thiopheneacetic acid.
Drozdzewski P, et al.
Polyhedron, 23(10), 1785-1792 (2010)
Study of Adsorption of 2-Thiopheneacetic Acid on Three Adsorbent Resins.
Wei R, et al.
Acta Polymerica Sinica / Gao Fen Zi Xue Bao, 4, 471-477 (2004)
Hye-Seon Shin et al.
ACS applied materials & interfaces, 5(4), 1429-1435 (2013-01-23)
Rosette-like nanoscale Au materials were simply prepared through one-pot reduction of the AuCl₄⁻ precursor by 2-thiopheneacetic acid (2-TAA) without extra surface capping ligands at room temperature. 2-TAA underwent polymerization into polythiophene derivatives while the AuCl₄⁻ precursor was simultaneously reduced into
Manuel Temprado et al.
The journal of physical chemistry. A, 112(41), 10378-10385 (2008-09-26)
The enthalpies of formation in the condensed and gas states, Delta f H m degrees (cd) and Delta f H m degrees (g), of 2- and 3-thiopheneacetic acids were derived from their respective enthalpies of combustion in oxygen, measured by
Cláudia Sg Gomes et al.
Journal of chemical technology and biotechnology (Oxford, Oxfordshire : 1986), 93(7), 1901-1915 (2018-07-17)
The extraction of biopharmaceuticals from plasma and serum often employs overly complicated antiquated procedures that can inflict serious damage on especially prone protein targets and which afford low purification power and overall yields. This paper describes systematic development of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service