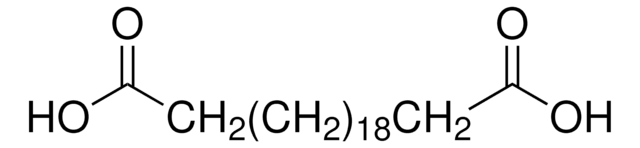177504
Hexadecanedioic acid
96%
Synonym(s):
Thapsic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOOC(CH2)14COOH
CAS Number:
Molecular Weight:
286.41
Beilstein:
1792831
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
96%
mp
120-123 °C (lit.)
SMILES string
OC(=O)CCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H30O4/c17-15(18)13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(19)20/h1-14H2,(H,17,18)(H,19,20)
InChI key
QQHJDPROMQRDLA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Hexadecanedioic acid (HDDA), also known as thapsic acid, is a straight-chain aliphatic dicarboxylic acid with the molecular formula C₁₆H30O₄. It features two carboxylic acid groups (-COOH) at each end of a saturated hydrocarbon chain, which imparts thermal stability, hydrophobicity, and biocompatibility to the polymers.
Application
Hexadecanedioic acid can be used:
- As a tracer (radiolabeled with Gallium-68), for cardiac metabolic imaging, enabling visualization of fatty acid metabolism in the heart during PET scans. Its biocompatibility and mimicry of natural fatty acid pathways enhance diagnostic assessments of cardiac function.
- As a precursor to prepare noncross-linked terpolyester via melt polycondensation. This material can be used to fabricate ecofriendly food packaging materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vukic Soskic et al.
Journal of proteome research, 5(12), 3453-3458 (2006-12-02)
Serum and plasma are the major sources of human material for clinical molecular diagnostics and drug discovery. However, due to the high abundance of some proteins, of which serum albumin (SA) is most prominent, lower-abundance proteins often remain undetectable in
J E Pettersen et al.
Journal of lipid research, 15(6), 551-556 (1974-11-01)
The activation of hexadecanedioic acid has been studied in subcellular fractions of human liver. The activation capacity in a total homogenate of human liver was found to be 0.5 micro mole/min/g wet wt of tissue, about 10% of that for
Anupam Mathur et al.
Bioorganic & medicinal chemistry, 16(17), 7927-7931 (2008-08-15)
Development of a (99m)Tc-fatty acid analogue is of interest, as (99m)Tc is logistically advantageous over the cyclotron-produced (11)C and (123)I. Synthesis of a 16 carbon fatty acid derivative and its radiolabeling with the novel [(99m)TcN(PNP)](2+) core is described here. Hexadecanedioic
N Shirane et al.
Biochemistry, 32(49), 13732-13741 (1993-12-14)
Cytochrome P450BM-3 preferentially oxidized fatty acids with terminal double or triple bonds to the omega-2 hydroxylated fatty acids rather than, respectively, to the epoxide or diacid metabolites. The enzyme is inactivated during catalytic turnover of long, terminally unsaturated fatty acids
Young-Jae You et al.
European journal of medicinal chemistry, 39(2), 189-193 (2004-02-28)
Esters of 4'-demethyl-4-deoxypodophyllotoxin (DDPT) with alkanoic acids and alkanedioic acids were prepared and tested for cytotoxic and antitumor activity. Among 19 esters, esters of propanoic acid, tetradecanedioic acid, 13-carboxyundecanoic acid, and hexadecanedioic acid improved the antitumor activity compared with that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service