100625
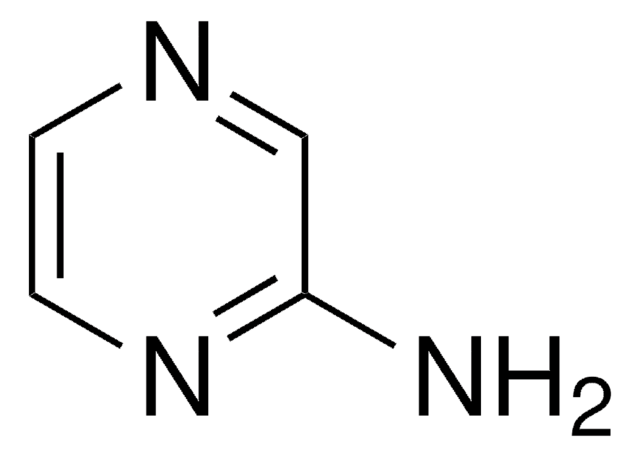
3-Amino-1,2,4-triazine
97%
Synonym(s):
3-Amino-as-triazine, [1,2,4]Triazin-3-ylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H4N4
CAS Number:
Molecular Weight:
96.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
174-177 °C (lit.)
SMILES string
Nc1nccnn1
InChI
1S/C3H4N4/c4-3-5-1-2-6-7-3/h1-2H,(H2,4,5,7)
InChI key
MJIWQHRXSLOUJN-UHFFFAOYSA-N
Related Categories
General description
3-amino-1,2,4-triazine (ATN) acts as a single molecular carbon nitride precursor during the preparation of mesoporous carbon nitride with high nitrogen content via polymerization. Additionally, it is also used in the synthesis of organometal complexes.
Application
- Antitumor activity in pharmaceutical applications: A study reported the development of a new library of 3-Amino-1,2,4-Triazine derivatives as PDK1 inhibitors, showing significant antitumor activity against pancreatic ductal adenocarcinoma, indicating its potential in cancer therapeutics (Carbone et al., 2023).
- Structural analysis in crystallography: The crystal structure and Hirshfeld surface analysis of a complex involving 3-Amino-1,2,4-triazine was detailed, providing insights into the molecular interactions and potential applications in materials science and coordination chemistry (Sangeetha et al., 2018).
- Catalytic applications in NMR technology: 3-Amino-1,2,4-triazine was used in a study to achieve significant NMR polarization in water using the SABRE technique, demonstrating its role as a catalyst in enhancing NMR sensitivity and its utility in magnetic resonance imaging (Zeng et al., 2014).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Crystal structure of 3-amino-1,2,4-triazin-5(2H)-one.
Long-Chih Hwang et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 18(6), 723-724 (2002-06-27)
P A Taheri et al.
The Journal of clinical investigation, 93(1), 147-154 (1994-01-01)
Ultrasonic probes were placed around dog femoral arteries to record blood flow. Hind paw scalding with boiling water (5 s) caused a marked increase in ipsilateral femoral blood flow that persisted for the 2-h observation period. Contralateral femoral blood flow
S S Greenberg et al.
Life sciences, 57(21), 1949-1961 (1995-01-01)
We evaluated the effect of in vivo and in vitro administration of nitro-containing and nitro-deficient L-arginine-derived nitric oxide (NO) synthase inhibitors on the measurement of NO in plasma, urine and HEPES buffered physiologic salt solution (PSS) by ozone chemiluminescence and
Grant Abernethy et al.
Journal of chromatography. A, 1285, 165-167 (2013-03-12)
A rapid liquid chromatography-mass spectrometry method to detect 3-amino-1,2,4-triazine (ATZ) in milk was developed as part of a programme to set up methods for detecting the economically motivated adulteration of raw milk with nitrogen-containing compounds. When ATZ was added to
Jirí Hanusek et al.
Organic & biomolecular chemistry, 5(3), 478-484 (2007-01-26)
Contrary to a previous report, the sulfurisation of phosphorus(III) derivatives by 3-amino-1,2,4-dithiazole-5-thione (xanthane hydride) does not yield carbon disulfide and cyanamide as the additional reaction products. The reaction of xanthane hydride with triphenyl phosphine or trimethyl phosphite yields triphenyl phosphine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service