C5421
Cholesterol Oxidase from microorganisms
aqueous solution, ≥30 units/mg protein (biuret)
Synonym(s):
Cholesterol:oxygen oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
aqueous solution
specific activity
≥30 units/mg protein (biuret)
mol wt
62 kDa
solubility
50 mM potassium phosphate buffer, pH 7.0: soluble (Cold)
shipped in
dry ice
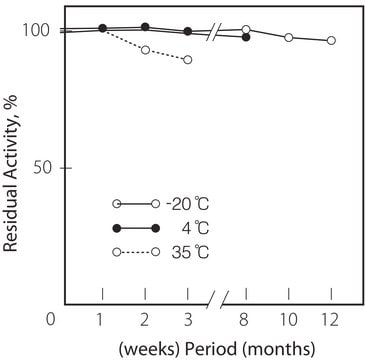
storage temp.
−70°C
Application
Cholesterol oxidase has been used in a study to improve treatment of oxysterol-mediated cytotoxicity. Cholesterol oxidase has also been used in a study that determined that cholesterol depletion impairs coupling between channel opening and vesicle release by allowing voltage-gated calcium channels to move further from release sites.
Cholesterol oxidase has been used in a study to improve treatment of oxysterol-mediated cytotoxicity. Cholesterol oxidase has also been used in a study that evaluated the effects of cholesterol depletion. This study reported that cholesterol depletion impairs the coupling between channel opening and vesicle release. Additionally, cholesterol oxidase is used to determine serum cholesterol levels. The enzyme also finds application in the microanalysis of steroids in food samples. It has also been used for distinguishing 3-ketosteroids from 3β-hydroxysteroids. Transgenic plants expressing cholesterol oxidase have been investigated in the fight against the cotton boll weevil. CHOD has also been used as a molecular probe to elucidate cellular membrane structures.
Biochem/physiol Actions
Cholesterol oxidase (CHOD) is a monomeric flavoprotein containing FAD that catalyzes the first step in cholesterol catabolism. This bifunctional enzyme oxidizes cholesterol to cholest-5-en-3-one in an FAD-requiring step. The pH optimum of the enzyme is 7.0 to 7.5 and temperature optimum is 50 °C. The pH stability is 5.7-7.8. Hg2+, Ag+, and ionic detergents inhibit the enzyme activity. Molecular mass of the enzyme is 62 kDa. Pathogenic bacteria require CHOD to infect a host′s macrophage.
Unit Definition
One unit will convert 1.0 μmole of cholesterol to 4-cholesten-3-one per min at pH 7.5 at 25 °C. Note: 4-cholesten-3-one may undergo isomerization.
Preparation Note
CHOD is soluble in cold 50 mM potassium phosphate buffer, pH 7.0. Prepare solutions immediately before use.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ruchi Chaube et al.
Biochimica et biophysica acta, 1821(2), 313-323 (2011-11-09)
Chronic exposure of blood vessels to cardiovascular risk factors such as free fatty acids, LDL-cholesterol, homocysteine and hyperglycemia can give rise to endothelial dysfunction, partially due to decreased synthesis and bioavailability of nitric oxide (NO). Many of these same risk
Laura Caldinelli et al.
The Journal of biological chemistry, 280(24), 22572-22581 (2005-04-09)
Cholesterol oxidase from Brevibacterium sterolicum is a monomeric flavoenzyme catalyzing the oxidation and isomerization of cholesterol to cholest-4-en-3-one. This protein is a class II cholesterol oxidases, with the FAD cofactor covalently linked to the enzyme through the His(69) residue. In
Porntip H Lolekha et al.
Clinica chimica acta; international journal of clinical chemistry, 339(1-2), 135-145 (2003-12-23)
Cholesterol oxidase is used for the determination of serum cholesterol. It can be derived from Streptomyces, Pseudomonas fluorescens, Cellulomonas, and Brevibacterium. This study compared the performance characteristics of four enzymes in the endpoint cholesterol determination. Using the Mega analyzer, we
Charlotte Ford et al.
PLoS pathogens, 14(5), e1007051-e1007051 (2018-05-05)
Pathogens hijack host endocytic pathways to force their own entry into eukaryotic target cells. Many bacteria either exploit receptor-mediated zippering or inject virulence proteins directly to trigger membrane reorganisation and cytoskeletal rearrangements. By contrast, extracellular C. trachomatis elementary bodies (EBs)
Yan Sun et al.
Biotechnology letters, 33(10), 2049-2055 (2011-06-28)
Site-directed mutagenesis was applied to enhance the thermostability and enzymatic activity of cholesterol oxidase (ChOx) isolated from Brevibacterium sp. Three amino acid residues (Q153E, F128L, and S143H) located near the FAD-binding site of the enzyme were substituted based on structural
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service