C2389
Cerulenin
from Cephalosporium caerulens, ≥98% (HPLC), powder, de novo phospholipid synthesis inhibitor
Synonym(s):
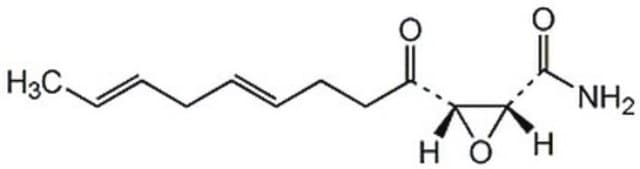
Helicocerin, (2R,3S,E,E)-2,3-Epoxy-4-oxo-7,10-dodecadienamide
About This Item
Recommended Products
product name
Cerulenin, ≥98% (HPLC), from Cephalosporium caerulens
biological source
Cephalosporium caerulens
Quality Level
Assay
≥98% (HPLC)
form
powder
mp
93 °C
solubility
acetone: 19.60-20.40 mg/mL, clear to slightly hazy, colorless to yellow
antibiotic activity spectrum
fungi
Mode of action
enzyme | inhibits
storage temp.
−20°C
SMILES string
C/C=C/C/C=C/CCC([C@@H]1[C@H](C(N)=O)O1)=O
InChI
1S/C13H18O3/c1-3-4-5-6-7-8-9-11(15)13-12(16-13)10(2)14/h3-4,6-7,12-13H,5,8-9H2,1-2H3/b4-3+,7-6+/t12-,13+/m0/s1
InChI key
PTNNGEBMCNMENY-JIVMHGEESA-N
Gene Information
human ... FASN(2194)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a fatty acid synthase inhibitor to study its effects on aldosterone-induced trained immunity
- as a blocker of fatty acid synthase to study its effects on the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2)
- as a supplement in yeast extract–peptone–dextrose/glycerol (YPD/G) agar plates for the isolation of cerulenin-resistant yeast strains
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cholesterol synthesis regulation by dietary levels, LDL receptors control lipid-rich LDL particle transport in cells.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Discover Bioactive Small Molecules for Lipid Signaling Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service