Y0000319
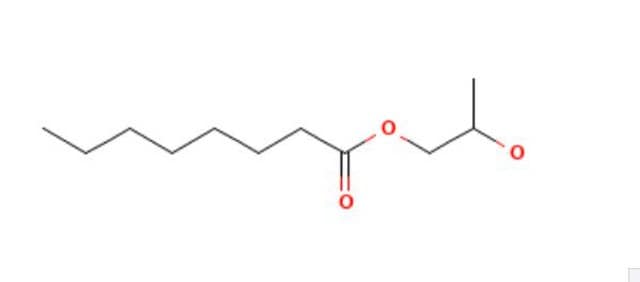
Propylene glycol monolaurate
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H30O3
CAS Number:
Molecular Weight:
258.40
EC Number:
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
propylene glycol
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C15H30O3/c1-3-4-5-6-7-8-9-10-11-12-15(17)18-13-14(2)16/h14,16H,3-13H2,1-2H3
InChI key
BHIZVZJETFVJMJ-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Propylene glycol monolaurate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B R Jasti et al.
The journal of investigative dermatology. Symposium proceedings, 3(2), 128-130 (1998-09-12)
Excipients are often used in transdermal formulations to overcome the formidable barrier offered by the epidermis in order to achieve the target flux. In this study we describe the use of frequency-domain fluorescence spectroscopy to characterize the effect of two
G D Irion et al.
Pharmaceutical research, 12(11), 1618-1622 (1995-11-01)
To measure the effect of a combination of excipients from a silicone based pressure sensitive adhesive (PSA) on drug transport across skin. Partitioning of propylene glycol monolaurate (PG-ML) from silicone PSA and a solution formulation into the stratum corneum (SC)
Nicholas C Obitte et al.
AIDS research and therapy, 10(1), 14-14 (2013-06-01)
CSIC (5-chloro-3-phenylsulfonylindole-2-carboxamide), a non-nucleoside reverse transcriptase inhibitor (NNRTI) has not been advanced as a therapeutic anti-HIV candidate drug due to its low aqueous solubility and poor bioavailability. The objective of this work was to formulate CSIC into self-emulsifying oil formulations
Archita Patel et al.
Current drug delivery, 12(6), 745-760 (2015-03-04)
The solid-self nanoemulsifying drug delivery system (S-SNEDDS) of Amiodarone hydrochloride (AH) was prepared and evaluated. AH exhibits poor aqueous solubility (0.3-0.5 mg/ml) and therefore variable oral bioavailability. Capmul MCM, Cremophor RH-40 and Propylene glycol were identified as oil, surfactant and
Rana Abu-Huwaij et al.
Drug development and industrial pharmacy, 33(4), 437-448 (2007-05-25)
The aim of this study was to develop a controlled release buccal mucoadhesive delivery system for systemic delivery of lidocaine hydrochloride as a model drug. In vitro release and buccal permeation as well as in vivo permeation of LDHCL patches
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service