860820P
Avanti
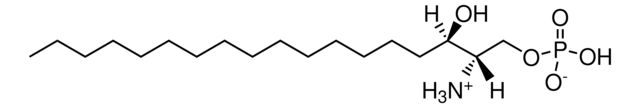
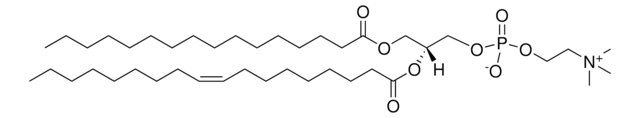
20:0(2S-OH) Ceramide
Avanti Research™ - A Croda Brand 860820P, powder
Synonym(s):
N-(2′-(S)-hydroxyarachidoyl)-D-erythro-sphingosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C38H75NO4
CAS Number:
Molecular Weight:
610.01
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 5 mg (860820P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860820P
lipid type
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCC/C=C/[C@@H](O)[C@@H](NC([C@@H](O)CCCCCCCCCCCCCCCCCC)=O)CO
General description
Ceramides linked to 2-hydroxy fatty acids (hFA) are present in the surface epithelium of the skin. 20:0(2S-OH) Ceramide is a unique ceramide containing 20C long chain base fatty acid (arachidic acid)-with 2′-hydroxyl group in S configuration.
Biochem/physiol Actions
Hydroxy fatty acid (hFA)-sphingolipids help in formation and function of myelin. In addition, they also play a vital role in cell signaling, cell differentiation and apoptosis. In epidermis, hFA-ceramides aid in permeability barrier function. NAD(P)H-dependent enzyme, fatty acid 2-hydroxylase (FA2H) catalyzes the synthesis of hFA-ceramides. FA2H gene mutation leads to the development of neurological disorders such as leukodystrophy and spastic paraparesis in humans. hFA-ceramides help PM02734 (elisidepsin), an antitumor drug to exhibit its activity.
Packaging
5 mL Amber Glass Screw Cap Vial (860820P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yi-He Ling et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 17(16), 5353-5366 (2011-06-22)
PM02734 (elisidepsin) is a synthetic marine-derived cyclic peptide of the kahalalide family currently in phase II clinical development. The mechanisms of cell death induced by PM02734 remain unknown. Human non-small-cell lung cancer (NSCLC) cell lines H322 and A549 were used
PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase
Ling YH, et al.
Clinical Cancer Research, 17(16), 5353-5366 (2011)
Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers
Guo L, et al.
Journal of Lipid Research, 53(7), 1327-1335 (2012)
Normal fur development and sebum production depends on fatty acid 2-hydroxylase expression in sebaceous glands
Maier H, et al.
The Journal of Biological Chemistry, 286(29), 25922-25934 (2011)
Helena Maier et al.
The Journal of biological chemistry, 286(29), 25922-25934 (2011-06-02)
2-Hydroxylated fatty acid (HFA)-containing sphingolipids are abundant in mammalian skin and are believed to play a role in the formation of the epidermal barrier. Fatty acid 2-hydroxylase (FA2H), required for the synthesis of 2-hydroxylated sphingolipids in various organs, is highly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service