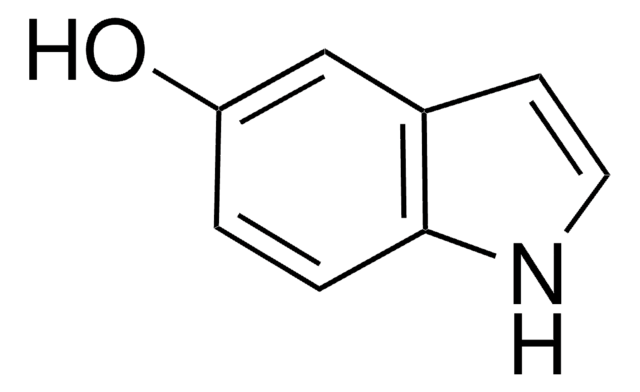
I5400
Indole-5-carboxylic acid
99%
Synonym(s):
5-Carboxyindole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
Beilstein:
124391
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
211-213 °C (lit.)
SMILES string
OC(=O)c1ccc2[nH]ccc2c1
InChI
1S/C9H7NO2/c11-9(12)7-1-2-8-6(5-7)3-4-10-8/h1-5,10H,(H,11,12)
InChI key
IENZCGNHSIMFJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Indole-5-carboxylic acid is an indole derivative. On electropolymerization, it affords electroactive polymer film of poly(indole-5-carboxylic-acid). Different concentrations of indole-5-carboxylic acid in sulfuric acid solution has been investigated for the preventive action against mild steel corrosion. On electropolymerization it affords a trimeric product. Characterization studies of the trimeric product by 1H NMR and various one- and two-dimensional NMR techniques have been reported.
Application
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles, as potential anticancer immunomodulators
- Reactant for preparation of indolyl-quinolines via metal- and solvent-free autoxidative coupling reaction
- Reactant for preparation of anthranilic acids using bromamine-B oxidant and palladium chloride catalyst
- Reactant for synthesis of indirubin derivatives
- Reactant for preparation of amide conjugates with ketoprofen, as inhibitors of Gli1-mediated transcription in Hedgehog pathway
- Reactant for preparation of piperazine-bisamide analogs as human growth hormone secretagogue receptor antagonists for treatment of obesity
Indole-5-carboxylic acid is the suitable reagent used to study the intramolecular excited state proton transfer in indole-2-carboxylic acid and indole-5-carboxylic acid in various solvents in acidic, basic, and neutral media by steady state and time resolved fluorescence spectroscopy. It may be used in the electrochemical synthesis of poly(indole-5-carboxylic acid) (PICA) films.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Inhibitive action of indole-5-carboxylic acid towards corrosion of mild steel in deaerated 0.5 M sulfuric acid solutions.
Quartarone G, et al.
Applied Surface Science, 252(23), 8251-8257 (2006)
Electropolymerisation of indole-5-carboxylic acid.
GordonaMackintosh J and Andrew R.
J. Chem. Soc., Faraday, 90(8), 1121-1125 (1994)
Characterization of the unsymmetrical trimer of indole-5-carboxylic acid by proton NMR spectroscopy.
Mackintosh JG, et al.
Magnetic Resonance in Chemistry, 32(9), 559-561 (1994)
Excited state proton transfer in indole-2-carboxylic acid and indole-5-carboxylic acid.
Bangal PR and Chakravorti S.
The Journal of Physical Chemistry A, 103(43), 8585-8594 (1999)
Electrodeposition of poly (indole-5-carboxylic acid) in boron trifluoride diethyl etherate containing additional diethyl ether.
Nie G, et al.
Electrochimica Acta, 52(24), 7097-7106 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[(3R)-3-Hydroxyhexanoyl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/529/281/b2c430b9-0eaf-42c2-9f83-291338e64187/640/b2c430b9-0eaf-42c2-9f83-291338e64187.png)