939633
Indoline-2-thione

≥-95%
Synonym(s):
2,3-Dihydro-1H-indole-2-thione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H7NS
CAS Number:
Molecular Weight:
149.21
MDL number:
UNSPSC Code:
12352100
Recommended Products
Quality Level
Assay
≥-95%
form
powder or crystals
reaction suitability
reaction type: Photocatalysis
color
white to yellow
SMILES string
S=C1NC2=CC=CC=C2C1
InChI
InChI=1S/C8H7NS/c10-8-5-6-3-1-2-4-7(6)9-8/h1-4H,5H2,(H,9,10)
InChI key
IGJWTYFTQNHSEK-UHFFFAOYSA-N
General description
Indoline-2-thione is a indole thiolate often used in the synthesis of (Indoline-2-S) related products.
Application
Indoline-2-thione is a indole thiolate has been used in:
- The MgI2-catalyzed nucleophilic ring opening of donor-acceptor cyclopropanes
- The synthesis of indole-fused dihydrothiopyrano scaffolds via [3+3] annulations of donor-acceptor cyclopropanes
- The preparation of 2-carboxylated thieno [2,3- b] indoles
- The synthesis of thioethers from aryl chlorides & alcohols
Features and Benefits
1-Methylindoline-2-thione is an affordable indole thiolate that is useful in general synthesis as well as the transition-metal free photocatalytic reduction of a range of strong polar C-F, C-O and C-Cl bonds.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Giacomo Mari et al.
Organic & biomolecular chemistry, 20(20), 4167-4175 (2022-05-10)
A metal-free strategy, alternative to the known complex cycloaddition reactions, towards 2-carboxylated thieno [2,3-b] indole derivatives has been successfully developed. The novel approach involves as starting materials easy accessible 1,2-diaza-1,3-dienes and indoline 2-thione and requires mild reaction conditions. Furthermore, the
Braj Gopal et al.
The Journal of organic chemistry, 88(1), 132-142 (2022-12-17)
A new methodology for the synthesis of N-haloindole-fused dihydrothiopyrano derivatives via (3 + 3)-annulation of donor-acceptor cyclopropanes (DACs) with indoline-2-thiones in the presence of Sc(OTf)3 as a Lewis acid catalyst has been developed. This protocol provides a variety of indole-fused
Pan Tang et al.
The Journal of organic chemistry, 87(16), 10890-10901 (2022-08-03)
MgI2-catalyzed nucleophilic ring-opening reactions of donor-acceptor cyclopropanes with indoline-2-thiones as easy-to-handle sulfur nucleophiles were investigated. A series of functionalized γ-indolylthio butyric acid derivatives were synthesized in good to excellent yields under mild reaction conditions. Furthermore, the thioether functionalized ring-opening products
Shuo Wu et al.
Journal of the American Chemical Society, 146(5), 2907-2912 (2024-01-24)
Thioethers, often found in pharmaceuticals and natural compounds, typically involve metal cross-coupling reactions, high temperatures, and the use of disagreeable thiols for their synthesis. Here we present a straightforward, thiol-free organocatalytic protocol that uses mild conditions to stitch together inexpensive
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service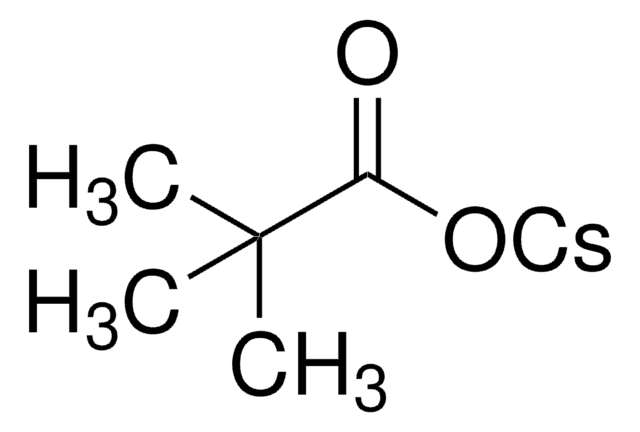


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)



