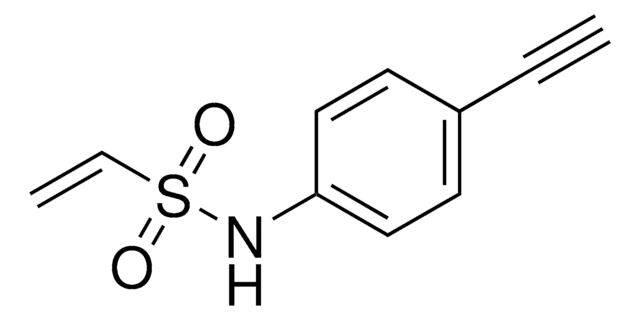
927538
AlkFAA-alkyne
≥95%
Synonym(s):
2-Fluoro-N-(hex-5-yn-1-yl)acrylamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H12FNO
Molecular Weight:
169.20
UNSPSC Code:
12352101
NACRES:
NA.21
Recommended Products
Application
AlkFAA-alkyne is a Michael acceptor probe that was designed to label cysteines, but is not very effective. The compound can be used as a negative control when run with other cysteine-reactive probes. A method was developed using cysteine-reactive compounds to allow for unbiased analysis of proteomic data in quantitative applications . The method uses light or heavy labeling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis . Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Profiling the proteome-wide selectivity of diverse electrophiles.
Zanon P RA, et al.
ChemRxiv : the preprint server for chemistry (2021)
Patrick R A Zanon et al.
Angewandte Chemie (International ed. in English), 59(7), 2829-2836 (2019-11-30)
Rapid development of bacterial resistance has led to an urgent need to find new druggable targets for antibiotics. In this context, residue-specific chemoproteomic approaches enable proteome-wide identification of binding sites for covalent inhibitors. Described here are easily synthesized isotopically labeled
Eranthie Weerapana et al.
Nature, 468(7325), 790-795 (2010-11-19)
Cysteine is the most intrinsically nucleophilic amino acid in proteins, where its reactivity is tuned to perform diverse biochemical functions. The absence of a consensus sequence that defines functional cysteines in proteins has hindered their discovery and characterization. Here we
Keriann M Backus et al.
Nature, 534(7608), 570-574 (2016-06-17)
Small molecules are powerful tools for investigating protein function and can serve as leads for new therapeutics. Most human proteins, however, lack small-molecule ligands, and entire protein classes are considered 'undruggable'. Fragment-based ligand discovery can identify small-molecule probes for proteins
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service