916080
Diethyl-4-cyclohexyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
≥95%
Synonym(s):
4-Alkyl-1,4-dihydropyridine reagent, DHP reagent for light-mediated His modification
About This Item
Recommended Products
Assay
≥95%
form
powder
storage temp.
−20°C
InChI
1S/C19H29NO4/c1-5-23-18(21)15-12(3)20-13(4)16(19(22)24-6-2)17(15)14-10-8-7-9-11-14/h14,17,20H,5-11H2,1-4H3
InChI key
GERWBKSVDHUVIT-UHFFFAOYSA-N
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis
A photocatalyst-free photo-induced denitroalkylation of ß-nitrostyrenes with 4-alkyl substituted Hantzsch esters at room temperature
Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis
Exploration of a chiral cobalt catalyst for visible-light-induced enantioselective radical conjugate addition
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service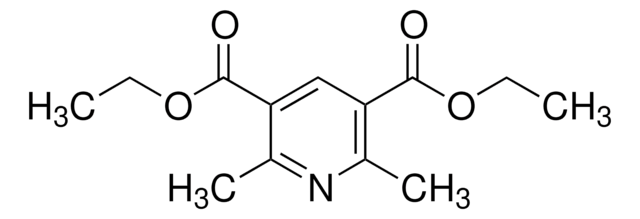






![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

![11H-Benzo[a]fluorene ≥98.0%](/deepweb/assets/sigmaaldrich/product/structures/373/290/b9e3f853-3649-464e-bfcc-a7152fa5b3b9/640/b9e3f853-3649-464e-bfcc-a7152fa5b3b9.png)
