446041
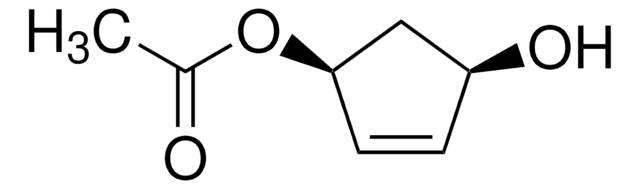
(1S,4R)-cis-4-Acetoxy-2-cyclopenten-1-ol
≥99%
Synonym(s):
(1R,3S)-(+)-cis-4-Cyclopentene-1,3-diol 1-acetate, (1R,3S)-4-Cyclopentene-1,3-diol 1-acetate, (1R,4S)-cis-4-Hydroxy-2-cyclopentenyl acetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H10O3
CAS Number:
Molecular Weight:
142.15
Beilstein:
4663992
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
solid
optical activity
[α]20/D +68°, c = 2.3 in chloroform
mp
49-51 °C (lit.)
SMILES string
CC(=O)O[C@@H]1C[C@H](O)C=C1
InChI
1S/C7H10O3/c1-5(8)10-7-3-2-6(9)4-7/h2-3,6-7,9H,4H2,1H3/t6-,7+/m1/s1
InChI key
IJDYOKVVRXZCFD-RQJHMYQMSA-N
Related Categories
Application
(1S,4R)-cis-4-Acetoxy-2-cyclopenten-1-ol can be used as:
- A building block for the synthesis of biologically significant carbocyclic nucleosides and prostaglandins.
- A starting material in the synthesis of azasugar analogs.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Theil, F. et al.
Journal of the Chemical Society. Perkin Transactions 1, 255-255 (1996)
(4R)-(+)-tert-Butyldimethylsiloxy-2-Cyclopenten-1-one: 2-Cyclopenten-1-one, 4-[[(1, 1-dimethylethyl) dimethylsilyl] oxy]-,(R)-
Paquette LA,
Organic Syntheses, 73(18), 36-36 (1996)
An improved preparation of highly enantiomerically enriched (R)-(+)-4-tert-butyldimethylsiloxy-2-cyclopenten-1-one
Myers AG, et al.
Tetrahedron Letters, 37(18), 3083-3086 (1996)
Facile synthesis of 9-[(1′ R, 2′ S)-2′-hydroxy-3′-oxocyclopentan-1′-yl]-9-H-adenine possessing inhibitory activity against human recombinant S-adenosyl-l-homocysteine hydrolase
Kitade Y, et al.
Tetrahedron Letters, 42(3), 433-435 (2001)
Paquette, L.A. et al.
Organic Syntheses, 73, 36-36 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service