All Photos(1)
About This Item
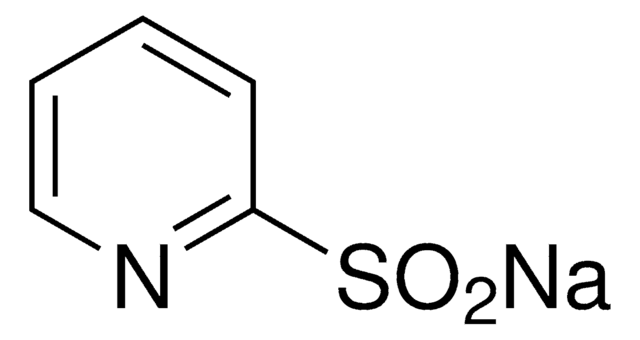
Linear Formula:
CH3CH=C(CO2CH3)2
CAS Number:
Molecular Weight:
158.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
refractive index
n20/D 1.447 (lit.)
density
1.111 g/mL at 25 °C (lit.)
SMILES string
COC(=O)\C(=C\C)C(=O)OC
InChI
1S/C7H10O4/c1-4-5(6(8)10-2)7(9)11-3/h4H,1-3H3
InChI key
FRCFZWCJSXQAMQ-UHFFFAOYSA-N
General description
Palladium (0)-catalyzed deconjugative allylation of dimethyl ethylidenemalonate has been reported.
Application
Dimethyl ethylidenemalonate has been employed:
- as electrophile in catalytic asymmetric Michael reactions of enamides and enecarbamates
- in preparation of N-bound α-cyanocarbanion complexes
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yoshihiro Sato et al.
The Journal of organic chemistry, 68(25), 9858-9860 (2003-12-06)
Palladium(0)-catalyzed deconjugative allylation of alkenylidenemalonates and alkylidenemalonates was achieved for the first time. Reactions of dimethyl 2-((E)-but-2-enylidene)malonate with various allylic acetates using LHMDS as a base in DMF in the presence of Pd(2)dba(3) (2.5 mol %) and PPh(3) (10 mol
Catalytic asymmetric Michael reactions with enamides as nucleophiles.
Florian Berthiol et al.
Angewandte Chemie (International ed. in English), 46(41), 7803-7805 (2007-09-05)
Carbon-carbon bond forming reactions of N-bound transition metal a-cyanocarbanions: a mechanistic probe for catalytic Michael reactions of nitriles
Naota T, et al.
Chemical Communications (Cambridge, England), 1, 63-64 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service