F0430
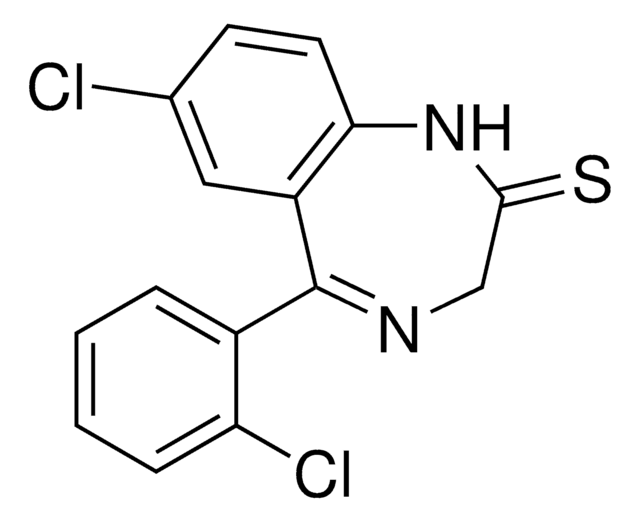
Fenobam
≥98% (HPLC), solid
Synonym(s):
N-(3-Chlorophenyl)-N′-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H11ClN4O2
CAS Number:
Molecular Weight:
266.68
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
solid
color
white
solubility
DMSO: >20 mg/mL
originator
Johnson & Johnson
storage temp.
2-8°C
SMILES string
CN1CC(=O)N=C1NC(=O)Nc2cccc(Cl)c2
InChI
1S/C11H11ClN4O2/c1-16-6-9(17)14-10(16)15-11(18)13-8-4-2-3-7(12)5-8/h2-5H,6H2,1H3,(H2,13,14,15,17,18)
InChI key
DWPQODZAOSWNHB-UHFFFAOYSA-N
Related Categories
Biochem/physiol Actions
Fenobam is a potent, selective, noncompetitive glutamate mGluR5 receptor antagonist. Fenobam displays inverse agonist properties; blocks mGluR5 constitutive activity in vitro (IC50 = 87 nM, slightly weaker than MPEP). Fenobam acts at an allosteric modulatory site shared with MPEP and binds the mGlu5 receptor with Kd values of 54 and 31 nM for rat and human receptors, respectively. Fenobam belongs to a structurally different class than MPEP; devoid of GABAergic activity and thus typical benzodiazepine-like side effects; displays anxiolytic activity.
Potent, selective, noncompetitive metabotropic glutamate receptor antagonist (mGluR5). Displays anxiolytic activity.
Features and Benefits
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glutamate Receptors (G Protein Family) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Johnson & Johnson. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
1H- and 13C-NMR spectra of fenobam.
G Pellizer et al.
Journal of pharmaceutical sciences, 72(2), 189-190 (1983-02-01)
Wolfgang Jacob et al.
Neuropharmacology, 57(2), 97-108 (2009-05-12)
Fenobam [N-(3-chlorophenyl)-N'-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea] was suggested to possess anxiolytic actions 30 years ago. Hoffmann-La Roche researchers recently reported that it is a selective and potent mGlu5 receptor antagonist, acting as a negative allosteric modulator. In the present study, we show that fenobam
Pari Malherbe et al.
Journal of neurochemistry, 98(2), 601-615 (2006-06-30)
Fenobam [N-(3-chlorophenyl)-N'-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea], a clinically validated non-benzodiazepine anxiolytic, has been shown to be a potent and non-competitive metabotropic glutamate (mGlu)-5 receptor antagonist. In the present study, we have used the site-directed mutagenesis coupled with three-dimensional receptor-based pharmacophore modelling to elucidate the
E Berry-Kravis et al.
Journal of medical genetics, 46(4), 266-271 (2009-01-08)
A pilot open label, single dose trial of fenobam, an mGluR5 antagonist, was conducted to provide an initial evaluation of safety and pharmacokinetics in adult males and females with fragile X syndrome (FXS). Twelve subjects, recruited from two fragile X
Daniella Rylander et al.
Neurobiology of disease, 39(3), 352-361 (2010-05-11)
L-DOPA remains the gold-standard treatment for Parkinson's disease but causes motor fluctuations and dyskinesia. Metabotropic glutamate receptor type 5 (mGluR5) has been proposed as a target for antidyskinetic therapies. Here, we evaluate the effects of fenobam, a noncompetitive mGluR5 antagonist
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service