30070
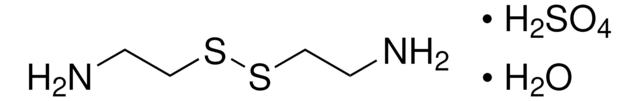
Cysteamine
≥98.0% (RT)
Synonym(s):
β-Mercaptoethylamine, 2-Aminoethanethiol, 2-Mercaptoethylamine, Decarboxycysteine, MEA, Thioethanolamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CH2SH
CAS Number:
Molecular Weight:
77.15
Beilstein:
635649
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (RT)
storage temp.
2-8°C
SMILES string
NCCS
InChI
1S/C2H7NS/c3-1-2-4/h4H,1-3H2
InChI key
UFULAYFCSOUIOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Cysteamine is suitable for use:
- in the preparation of cysteamine modified gold nanoparticles (AuNP)
- in the fabrication of SU-8 microrods, where in, the amine group of cysteamine reacts with the unreacted epoxide rings present on the surface of the particles, thereby opening it and forming a covalent bond
- to enhance in vitro development of porcine oocytes matured and fertilized in vitro
- in a study to demonstrate the depletion effect of cysteamine on cystinotic leucocyte granular fractions of cystine by disulphide interchange
- as a radioprotector
- to administer subcutaneously in rats to study its blocking effect on somatostatin secretion without modifying the pancreatic insulin or glucagon content
- as a scavenger in electrophoretic gels (acetic acid/urea gels)
Biochem/physiol Actions
Cysteamine (β-mercaptoethylamine) depletes cystine from patient′s cells and there by regulates renal glomerular function and increases growth in them. Therefore, cysteamine is considered to be a potential therapeutic for nephropathic cystinosis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Successful treatment of severe paracetamol overdosage with cysteamine.
L F Prescott et al.
Lancet (London, England), 1(7858), 588-592 (1974-04-06)
W A Gahl et al.
The Biochemical journal, 228(3), 545-550 (1985-06-15)
Cystinotic lysosome-rich leucocyte granular fractions, loaded with [35S]cystine, were exposed to different cystine-depleting agents. During a 30 min incubation at 37 degrees C, untreated cystinotic granular fractions lost negligible [35S]cystine when corrected for lysosome rupture. Granular fractions exposed to 0.1
R L Sorenson et al.
Diabetes, 32(4), 377-379 (1983-04-01)
Cysteamine (300 mg/kg) administered subcutaneously depletes pancreatic somatostatin to 36% of control levels, but does not alter pancreatic insulin or glucagon content. Although perfusion of pancreata from normal animals with glucose (300 mg/dl) markedly stimulated somatostatin release, pancreata from cysteamine-treated
M J Barratt et al.
Proceedings of the National Academy of Sciences of the United States of America, 91(11), 4781-4785 (1994-05-24)
Diverse agents, including growth factors and phorbol esters, induce rapid transcriptional activation of a subset of immediate-early (IE) genes that include the protooncogenes c-fos and c-jun. Among the earliest nuclear signaling events concomitant with IE gene activation is the phosphorylation
J Carmichael et al.
Cancer research, 47(4), 943-946 (1987-02-15)
Radiation survival curves were generated for V79 Chinese hamster and two human lung cancer cell lines (NCI-H460 and NCI-H249) with doubling times of 10, 20, and 85 h, respectively, using a standard clonogenic assay, a dye exclusion assay, and a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service