191698
2-Sulfobenzoic acid cyclic anhydride
technical grade, 90%
Synonym(s):
2,1-Benzoxathiol-3-one-1,1-dioxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H4O4S
CAS Number:
Molecular Weight:
184.17
Beilstein:
139893
EC Number:
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
powder
bp
184-186 °C/18 mmHg (lit.)
mp
116 °C
solubility
methanol: 50 mg/mL
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
O=C1OS(C2=CC=CC=C21)(=O)=O
InChI
1S/C7H4O4S/c8-7-5-3-1-2-4-6(5)12(9,10)11-7/h1-4H
InChI key
NCYNKWQXFADUOZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chengli Zu et al.
Rapid communications in mass spectrometry : RCM, 24(1), 120-128 (2009-12-10)
A derivatization procedure has been developed for the improved characterization of fatty alcohol ethoxylate non-ionic surfactants by liquid chromatography/mass spectrometry. The end hydroxyl group of each surfactant species was converted into an oxycarbonylbenzene-2-sulfonic acid group with 2-sulfobenzoic anhydride under mild
A Moulin et al.
Biochemistry, 28(15), 6340-6346 (1989-07-25)
2-Sulfobenzoic cyclic anhydride (SBA) rapidly and selectively inactivates porcine pancreatic lipase (PPL) only when added during the hydrolysis of an emulsified ester such as tributyrin or dodecyl acetate. The present data suggest that the inactivation of PPL occurs preferentially at
Christin Stegemann et al.
Rapid communications in mass spectrometry : RCM, 24(5), 599-604 (2010-02-16)
Two cyclic theta-defensin peptides were isolated from leukocytes of the hamadryas baboon, Papio hamadryas, and purified to homogeneity by gel electrophoresis and reversed-phase high-performance liquid chromatography. Both peptides had high in vitro activity against Escherichia coli, Listeria monocytogenes, methicillin-resistant Staphylococcus
Modification of epsilon-amino group of lysine in proteins by acylation with pyromellitic dianhydride and o-sulphobenzoic anhydride.
A Bagree et al.
FEBS letters, 120(2), 275-277 (1980-11-03)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service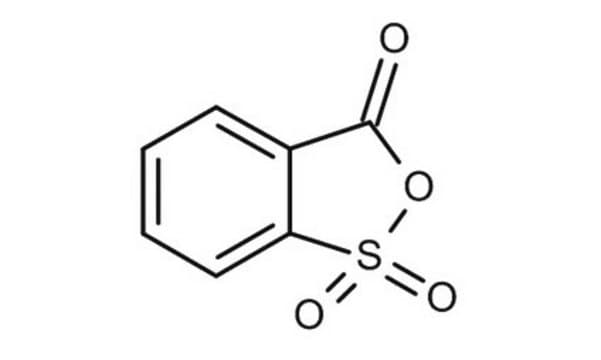

![N-[2-(Fmoc-amino)-ethyl]-Gly-O-tBu hydrochloride ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/641/926/3fedc773-b21f-4419-afd5-87e20df0156a/640/3fedc773-b21f-4419-afd5-87e20df0156a.png)










