I2800
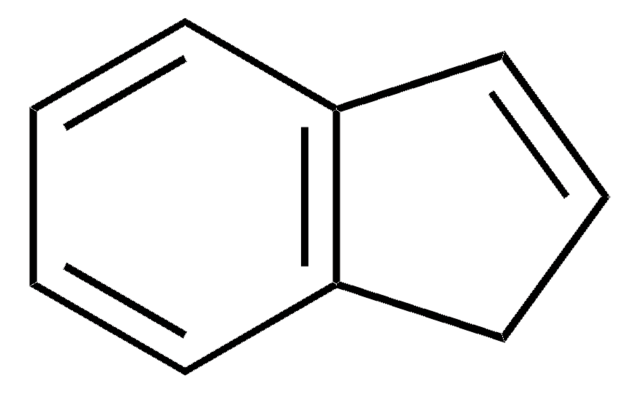
Indene
contains 50-100 ppm tert-butylcatechol as stabilizer, technical grade, ≥90%
Synonym(s):
Indonaphthene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8
CAS Number:
Molecular Weight:
116.16
Beilstein:
635873
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
≥90%
form
liquid
contains
50-100 ppm tert-butylcatechol as stabilizer
refractive index
n20/D 1.595 (lit.)
bp
181-182 °C (lit.)
mp
−5-−3 °C (lit.)
density
0.996 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=Cc2ccccc12
InChI
1S/C9H8/c1-2-5-9-7-3-6-8(9)4-1/h1-6H,7H2
InChI key
YBYIRNPNPLQARY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Two-step synthesis of stable dioxadicarbaporphyrins from bis(3-indenyl)methane.
Timothy D Lash et al.
Angewandte Chemie (International ed. in English), 51(43), 10871-10875 (2012-09-25)
Regioselective synthesis of indenols by rhodium-catalyzed C-H activation and carbocyclization of aryl ketones and alkynes.
Krishnamoorthy Muralirajan et al.
Angewandte Chemie (International ed. in English), 50(18), 4169-4172 (2011-03-31)
Adam C Glass et al.
Organic letters, 10(21), 4855-4857 (2008-10-07)
A new methodology for the preparation of substituted naphthalenes starting from readily available indenones, organometal reagents, and trimethylsilyldiazomethane via a catalytic rearrangement process is described. Hindered biaryl naphthalenes, including triortho-substituted biaryls, can be accessed through our method. Our results are
Cobalt-catalyzed regioselective synthesis of indenamine from o-iodobenzaldimine and alkyne: intriguing difference to the nickel-catalyzed reaction.
Chuan-Che Liu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(31), 9503-9506 (2008-09-24)
Ermitas Alcalde et al.
Organic & biomolecular chemistry, 6(20), 3795-3810 (2008-10-10)
A series of novel indene derivatives designed by a scaffold selection gave access to several examples of (Z)-arylmethylideneindenes and indenylsulfonamides that acted as serotonin 5-HT(6) receptor ligands. Different synthetic multistep routes could be applied to these target compounds, each with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service