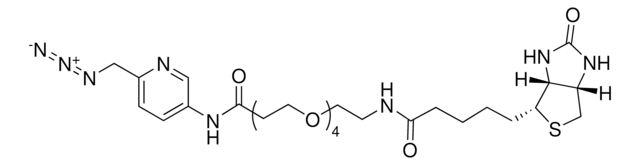
924229
(S,R,S)-AHPC-Me
95%
Synonym(s):
(2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-((S)-1-(4-(4-methylthiazol-5-yl)phenyl)ethyl)pyrrolidine-2-carboxamide, PROTAC® research ligand, VH032 methyl derivative
About This Item
Recommended Products
ligand
VH032
Quality Level
Assay
95%
form
powder
storage temp.
2-8°C
SMILES string
C([C@H](C(C)(C)C)N)(=O)N1[C@H](C(N[C@@H](C)C2=CC=C(C=C2)C3=C(C)N=CS3)=O)C[C@@H](O)C1
Application
Related Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Browse our growing synthesis and research tools: Protein Degrader Building Blocks
Other Notes
Discovery of ARD-69 as a highly potent proteolysis targeting chimera (PROTAC) degrader of androgen receptor (AR) for the treatment of prostate cancer
Design, Synthesis, and Biological Evaluation of MEK PROTACs
Antibody–PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4
Discovery of a First-in-Class Mitogen-Activated Protein Kinase Kinase 1/2 Degrader
Discovery of PROTAC BCL-XL degraders as potent anticancer agents with low on-target platelet toxicity
Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression
A caged E3 ligase ligand for PROTAC-mediated protein degradation with light
Discovery of SHP2-D26 as a First, Potent, and Effective PROTAC Degrader of SHP2 Protein
Selective CDK6 degradation mediated by cereblon, VHL, and novel IAP-recruiting PROTACs
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service