901112
PhenN O-PC™ B0301
New Iridium, ≥97%
Synonym(s):
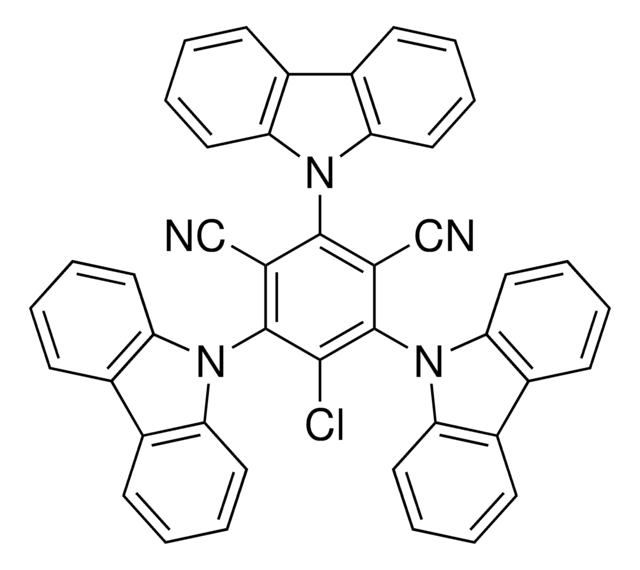
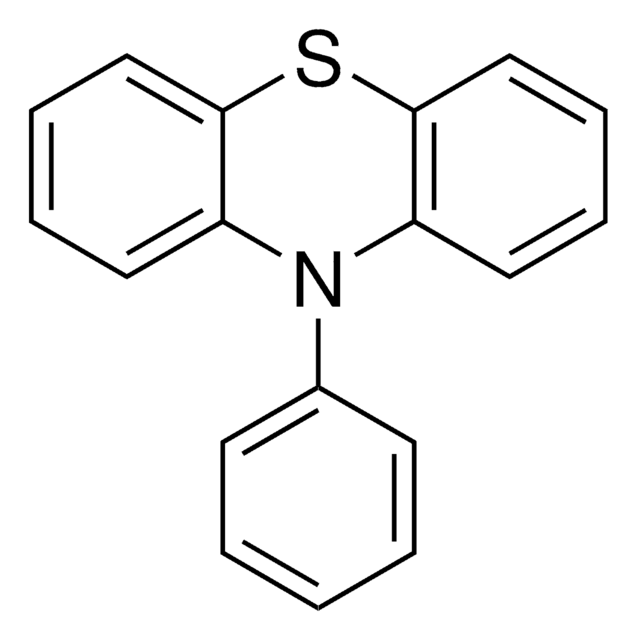
Miyake polymerization organophotoredox catalyst, 5,10-Di(2-Naphthyl)-5,10-dihydrophenazine, PhenN_2Naph
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C32H22N2
CAS Number:
Molecular Weight:
434.53
UNSPSC Code:
12161600
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
powder or crystals
reaction suitability
reagent type: catalyst
reaction type: Photocatalysis
photocatalyst activation
460 nm
Related Categories
Application
This dihydrophenazine-based organic photoredox catalyst (in addition to the dihydrophenazine catalyst 901111) was designed to be a strong excited-state reductant and possesses advanced photophysical and electrochemical properties, enabling it to serve as a sustainable replacement for ruthenium- or iridium-based photoredox catalysts. For example, dihydrophenazine and phenoxazine derivatives were demonstrated to replace ruthenium or iridium complexes in the application of photoredox-catalyzed atom transfer radical polymerization (ATRP) for controlled polymer synthesis and small molecule transformations such as trifluoromethylation, atom transfer radical addition, and dual Nickel/photoredox catalyzed C-N and C-S cross-couplings. Dihydrophenazine- and phenoxazine-based organic photoredox catalysts were introduced in collaboration with the Miyake Research Group.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Strongly Reducing Visible Light Organic Photoredox Catalysts as Sustainable Alternatives to Precious Metals
Organocatalyzed Atom Transfer Radical Polymerization Driven by Visible Light
Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts
Intramolecular Charge Transfer and Ion Pairing in N, N-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization
Organocatalyzed Atom Transfer Radical Polymerization Driven by Visible Light
Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts
Intramolecular Charge Transfer and Ion Pairing in N, N-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization
Legal Information
Patent application PCT/US2016/058245. Sold in collaboration with New Iridium Inc. For orders greater than 25g, please contact New Iridium at chern@newiridium.com or visit https://www.newiridium.com.
PhenN O-PC is a trademark of New Iridium Inc.
Phenox O-PC is a trademark of New Iridium LLC
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ya Du et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(46), 10962-10968 (2017-06-28)
Photoredox catalysis is a versatile approach for the construction of challenging covalent bonds under mild reaction conditions, commonly using photoredox catalysts (PCs) derived from precious metals. As such, there is need to develop organic analogues as sustainable replacements. Although several
Jordan C Theriot et al.
Science (New York, N.Y.), 352(6289), 1082-1086 (2016-04-02)
Atom transfer radical polymerization (ATRP) has become one of the most implemented methods for polymer synthesis, owing to impressive control over polymer composition and associated properties. However, contamination of the polymer by the metal catalyst remains a major limitation. Organic
Chern-Hooi Lim et al.
Journal of the American Chemical Society, 139(1), 348-355 (2016-12-16)
Photoexcited intramolecular charge transfer (CT) states in N,N-diaryl dihydrophenazine photoredox catalysts are accessed through catalyst design and investigated through combined experimental studies and density functional theory (DFT) calculations. These CT states are reminiscent of the metal to ligand charge transfer
Ryan M Pearson et al.
Journal of the American Chemical Society, 138(35), 11399-11407 (2016-08-25)
N-Aryl phenoxazines have been synthesized and introduced as strongly reducing metal-free photoredox catalysts in organocatalyzed atom transfer radical polymerization for the synthesis of well-defined polymers. Experiments confirmed quantum chemical predictions that, like their dihydrophenazine analogs, the photoexcited states of phenoxazine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![10-Ethyl-3,7,8-trimethyl-benzo[g]pteridine-2,4(3H,10H)-dione ≥95%](/deepweb/assets/sigmaaldrich/product/structures/610/004/664b92ed-0ce1-4e66-9f3e-d1cc53f1bc9a/640/664b92ed-0ce1-4e66-9f3e-d1cc53f1bc9a.png)