900889
Lithium phenyl-2,4,6-trimethylbenzoylphosphinate
≥95%
Synonym(s):
LAP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H16LiO3P
CAS Number:
Molecular Weight:
294.21
MDL number:
UNSPSC Code:
12352128
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥95%
form
crystalline powder
color
white to off-white
storage temp.
2-8°C
SMILES string
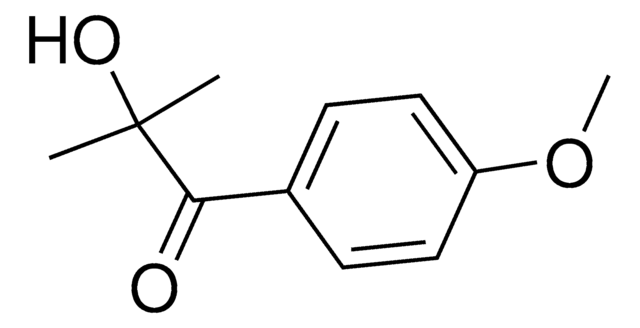
CC1=C(C(P(C2=CC=CC=C2)(O[Li])=O)=O)C(C)=CC(C)=C1
Looking for similar products? Visit Product Comparison Guide
Application
Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) is a water soluble, cytocompatible, Type I photoinitiator for use in the polymerization of hydrogels or other polymeric materials. This photoinitator is preferred over Irgacure 2959 for biological applications due to its increased water solubility, increased polymerization rates with 365 nm light, and absorbance at 400 nm allowing for polymerization with visible light. The improved polymerization kinetics enable cell encapsualation at reduced initiator concentration and longer wavelength light, which has been shown to reduce initiator toxicity and increase cell viability.
Features and Benefits
- Superior water solubility
- Biocompatible
- Sensitiveto visible light
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tiffany Zhang et al.
Scientific reports, 10(1), 15796-15796 (2020-09-27)
Inspired by the interesting natural antimicrobial properties of honey, biohybrid composite materials containing a low-fouling polymer hydrogel network and an encapsulated antimicrobial peroxide-producing enzyme have been developed. These synergistically combine both passive and active mechanisms for reducing microbial bacterial colonization.
Joshua D McCall et al.
Biomacromolecules, 13(8), 2410-2417 (2012-06-30)
Photoinitiated polymerization remains a robust method for fabrication of hydrogels, as these reactions allow facile spatial and temporal control of gelation and high compatibility for encapsulation of cells and biologics. The chain-growth reaction of macromolecular monomers, such as acrylated PEG
Zhiguang Qiao et al.
Biomaterials, 266, 120385-120385 (2020-10-30)
Despite significant advances in osteochondral tissue engineering, it remains challenging to successfully reconstruct native-like complex tissues organized in three-dimension with spatially varying compositional, structural and functional properties. In this contribution, inspired by the gradients in extracellular matrix (ECM) composition and
Kavin Kowsari et al.
iScience, 24(11), 103372-103372 (2021-11-27)
To address current unmet needs in terms of scalability and material biocompatibility for future photocrosslinking-based additive manufacturing technologies, emergent platform designs are in inexorable demand. In particular, a shift from the present use of cell-damaging UV light sources in light-based
Devon A Bowser et al.
Biofabrication, 12(1), 015002-015002 (2019-09-06)
The high attrition rate of neuro-pharmaceuticals as they proceed to market necessitates the development of clinically-relevant in vitro neural microphysiological systems that can be utilized during the preclinical screening phase to assess the safety and efficacy of potential compounds. Historically
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service