All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H8O4
CAS Number:
Molecular Weight:
192.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
296-299 °C (lit.)
SMILES string
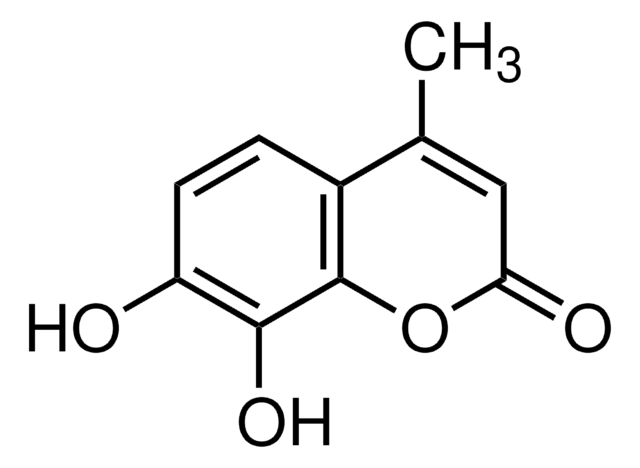
CC1=CC(=O)Oc2cc(O)cc(O)c12
InChI
1S/C10H8O4/c1-5-2-9(13)14-8-4-6(11)3-7(12)10(5)8/h2-4,11-12H,1H3
InChI key
QNVWGEJMXOQQPM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5,7-Dihydroxy-4-methylcoumarin can be obtained by reacting phloroglucinol and ethylacetoacetate via Pechmann condensation in the presence of sulfuric acid.
Application
5,7-Dihydroxy-4-methylcoumarin may be used in the synthesis of pyrano[2,3-h]coumarin derivatives and 5,7-dihydroxy-8-formyl-4-methylcoumarin.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Modified coumarins. 27. Ssynthesis and antioxidant activity of 3-substituted 5, 7-dihydroxy-4-methylcoumarins.
Garazd MM, et al.
Chemistry of Natural Compounds, 43(1), 19-23 (2007)
A one-pot, three-component synthesis of new pyrano [2, 3-h] coumarin derivatives.
Karami B, et al.
Tetrahedron Letters, 53(12), 1445-1446 (2012)
Formylation of Benzopyrones. II. Formylation of Hydroxycoumarins with N-Methylformanilide1.
Naik RM and Thakor VM.
The Journal of Organic Chemistry, 22(12), 1630-1633 (1957)
Etil Guzelmeric et al.
Journal of pharmaceutical and biomedical analysis, 132, 35-45 (2016-10-04)
Chamomile tea composed of dried flower heads of Matricaria recutita L. (Asteraceae) is one of the most popular single ingredient herbal teas. Tea industries, spice shops or public bazaars are mostly supplied chamomile as a raw material via cultivation or
Etil Guzelmeric et al.
Journal of pharmaceutical and biomedical analysis, 107, 108-118 (2015-01-13)
Brewed tea of chamomile flowers (Matricaria recutita L.) (Asteraceae) has been extensively consumed for centuries due to either its pleasant taste or medicinal purposes. On the other hand, the major problem is difficulty in distinguishing the genuine specimen when supplying
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service