All Photos(1)
About This Item
Empirical Formula (Hill Notation):
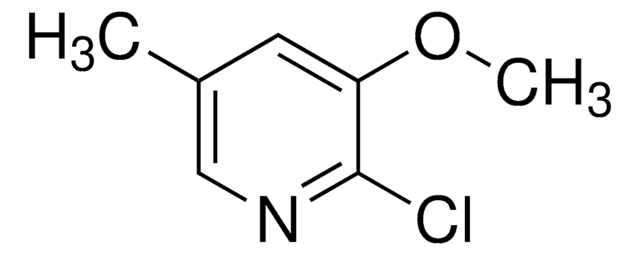
C6H6ClN
CAS Number:
Molecular Weight:
127.57
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.53 (lit.)
bp
97 °C/30 mmHg (lit.)
density
1.169 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
Cc1ccc(Cl)nc1
InChI
1S/C6H6ClN/c1-5-2-3-6(7)8-4-5/h2-4H,1H3
InChI key
VXLYOURCUVQYLN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Chloro-5-methylpyridine is a pesticide intermediate. Its synthesis by many methods has been reported.
Application
2-Chloro-5-methylpyridine may be used in the synthesis of:
- 2-methylthio-5-pyridinemethylene amine
- 5-methyl-2,2′-bipyridine
- 1-(5′-methyl-2,2′-bipyridin-5-yl)-2,5-dimethyl-1H-pyrrole
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and herbicidal activity of (Z)-ethoxyethyl 2-cyano-3-(2-methylthio-5-pyridylmethylamino) acrylates.
Wang QM, et al.
Heteroatom Chem., 15(1), 67-70 (2004)
The preparation of 2-chloro-5-methyl-pyridine in airlift loop reactor.
Jianping W, et al.
Chemical Engineering Journal, 95(1), 33-36 (2003)
Synthesis of Differently Disubstituted 2,2'-Bipyridines by a Modified Negishi Cross-Coupling Reaction.
Lutzen A, et al.
European Journal of Organic Chemistry, 2003(20), 3948-3957 (2003)
Synthesis of 5-Substituted 2,2'-Bipyridines from Substituted 2-Chloropyridines by a Modified Negishi Cross-Coupling Reaction.
Lutzen A and Hapke M.
European Journal of Organic Chemistry, 2002(14), 2292-2297 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service