All Photos(2)
About This Item
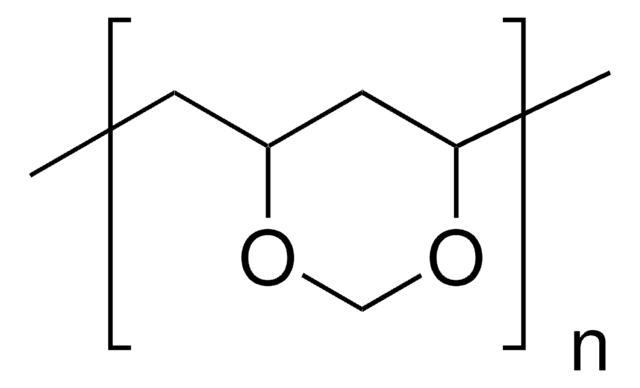
Linear Formula:
[CH2CH(O2CCH3)]n
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
beads
mol wt
average Mw ~500,000 by GPC
storage temp.
2-8°C
SMILES string
COC(=O)C=C
InChI
1S/C4H6O2/c1-3-6-4(2)5/h3H,1H2,2H3
InChI key
XTXRWKRVRITETP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Seon Tae Kim et al.
International journal of pediatric otorhinolaryngology, 77(1), 113-116 (2012-11-08)
The purpose of this prospective study was to determine the effectiveness of polyurethane foam (PUF) and polyvinyl acetate (PA) as packing materials for reducing post-conchotomy bleeding, pain, and headaches. This study was a prospective, randomized and single-blinded controlled study. Fifty-two
Dae Woo Kim et al.
American journal of rhinology & allergy, 26(5), e147-e149 (2012-11-22)
This study investigated the efficacy of glove finger-coated polyvinyl acetate (PA) pack on hemostasis, pain levels, and wound healing after endoscopic sinus surgery (ESS). A prospective, randomized, double-blinded controlled study was performed in 30 patients who underwent bilateral ESS for
Priscileila Colerato Ferrari et al.
Carbohydrate polymers, 91(1), 244-252 (2012-10-10)
In this work pellets containing chitosan for colonic drug delivery were developed. The influence of the polysaccharide in the pellets was evaluated by swelling, drug dissolution and intestinal permeation studies. Drug-loaded pellets containing chitosan as swellable polymer were coated with
Dima Ghanam et al.
International journal of pharmaceutics, 409(1-2), 9-18 (2011-02-22)
κ-Carrageenan is a novel pelletisation aid with high formulation robustness and quick disintegration leading to fast drug release unlike the matrix-like release from non-disintegrating microcrystalline cellulose pellets. Compression of pellets into tablets is cost effective. The feasibility of formulating multiparticulate
Packa Antovska et al.
Pharmaceutical development and technology, 18(2), 481-489 (2012-09-25)
Differential scanning calorimetry and Fourier transform infrared spectroscopy were applied as screening analytical methods to assess the solid-state compatibility of indapamide (4-chloro-N-(2-methyl-2,3-dihydroindol-1-yl)-3-sulfamoyl-benzamide) with several polymers aimed for development of 24 h sustained release solid-dosage formulation. After the initial research phase which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service